Enhanced elemental mercury removal using modified natural zeolites: a study on activation methods and adsorption stability
IF 4.7
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
The removal of elemental mercury (Hg0) from industrial gas streams remains a pressing environmental challenge. This study investigates the performance of natural zeolites modified via two activation methods – spraying and ion exchange – to enhance mercury adsorption efficiency. Samples (B1–B12, C1–C12) were functionalized using silver and iron precursors in nitrate and chloride forms. Their physicochemical properties were analysed using XRD, SEM-EDS mapping, and FTIR. Mercury removal efficiency was evaluated over six consecutive adsorption cycles using a custom-built laboratory scale measurement system. Twelve months after exposure, long-term mercury retention and potential re-emission were assessed with a direct mercury analyser. Results show that ion exchange, particularly with silver nitrate (C1–C3) and iron nitrate (C4–C6), led to more homogeneous precursor incorporation and higher Hg0 retention. The C6 sample exhibited the best overall performance. In contrast, the spraying method resulted in less uniform precursor distribution, although silver chloride-modified samples (B10–B12) also demonstrated high adsorption capacity. Nitrate-based precursors outperformed chloride counterparts in both efficiency and long-term stability. No direct relationship was found between zeolite grain size and mercury removal efficiency, indicating that internal porosity and precursor diffusion are more influential than particle dimensions. These findings highlight the critical role of activation strategy and precursor chemistry in optimizing zeolite-based sorbents. Ion exchange with nitrate precursors appears most promising for effective, stable mercury capture in industrial applications.
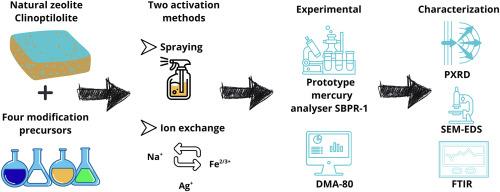
改性天然沸石增强单质汞去除:活化方法及吸附稳定性研究
从工业气体流中去除单质汞(Hg0)仍然是一项紧迫的环境挑战。研究了采用喷雾和离子交换两种活化方法对天然沸石进行改性,以提高其对汞的吸附效率。样品(B1-B12, C1-C12)以硝酸盐和氯化物形式用银和铁前体进行功能化。采用XRD、SEM-EDS作图和FTIR对其理化性质进行了分析。使用定制的实验室规模测量系统,通过六个连续的吸附循环来评估汞的去除效率。接触12个月后,用直接汞分析仪评估长期汞潴留和潜在的再排放。结果表明,离子交换,特别是与硝酸银(C1-C3)和硝酸铁(C4-C6)的离子交换,使前驱体掺入更均匀,Hg0保留率更高。C6样品表现出最好的综合性能。相比之下,喷涂方法导致前驱体分布不均匀,尽管氯化银修饰的样品(B10-B12)也表现出较高的吸附能力。硝酸盐基前驱体在效率和长期稳定性方面都优于氯化物。沸石颗粒尺寸与除汞效率无直接关系,表明内部孔隙度和前驱体扩散比颗粒尺寸影响更大。这些发现强调了活化策略和前驱体化学在优化沸石基吸附剂中的关键作用。离子交换与硝酸盐前体似乎最有希望有效,稳定的汞捕获在工业应用中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


