Synthesis of Core–Shell ETS-4@LSX zeolite composite to enhance CO2/N2 selectivity in flue gas separation
IF 4.7
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
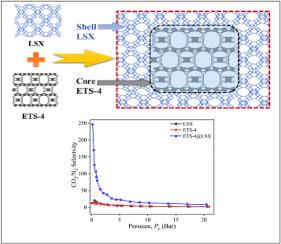
The selective removal of CO2 from the flue gas remains a quite challenging due to the weak selectivity of CO2/N2 in adsorbents. Therefore, it is necessary to design an effective sorbent to improve its selectivity. This work attempts to synthesis of novel core-shell ETS-4@LSX composite via a seed-assisted hydrothermal method. In this composite structure, a small pore titanosilcate ETS-4 as a core, while a large pore aluminosilicate LSX forms the outer shell. The effects of ETS-4 seed loading (1–5 wt.%) and crystallization time on the formation and structural integrity of the core–shell architecture was systematically investigated. The structural and physicochemical properties of samples were characterized by XRD, FE-SEM, HR-TEM, EDS, FT-IR, TGA, N2 adsorption-desorption, and pore size distribution. From XRD pattern and FE-SEM results confirmed that the composite synthesized with 2 wt% ETS-4 seed and 3 h crystallization time has pure phase of ETS-4@LSX structure. HR-TEM imaging revealed uniform growth of LSX over the ETS-4 surface, resulting in the formation of a continuous shell. The 2 wt% ETS-4@LSX composite demonstrated an outstanding CO2/N2 equilibrium selectivity of 81.4 at 1 bar and 303 K with five and seven times higher than that of pure LSX and ETS-4, respectively. At 20 bar, the composite achieved a CO2 uptake of 5.25 mmol g−1 and an N2 uptake of 0.50 mmol g−1. Dynamic adsorption study exhibited the 2 wt% ETS-4@LSX has 3.52 mmol g−1 CO2 sorption capacity and N2 uptake capacity of 0.26 mmol g−1. The enhanced adsorption capacity and selectivity of ETS-4@LSX are attributed to its dual-pore structure, highlighting its potential as an effective adsorbent for CO2 capture from flue gas.
核壳分子筛复合材料ETS-4@LSX的合成以提高烟气分离中CO2/N2的选择性
由于吸附剂中CO2/N2的选择性较弱,从烟气中选择性去除CO2仍然是一个相当具有挑战性的问题。因此,有必要设计一种有效的吸附剂来提高其选择性。本工作试图通过种子辅助水热法合成新型核壳ETS-4@LSX复合材料。在该复合结构中,以小孔钛硅酸盐ETS-4为核心,大孔铝硅酸盐LSX为外壳。系统研究了ETS-4种子负载(1-5 wt.%)和结晶时间对核壳结构形成和结构完整性的影响。采用XRD、FE-SEM、HR-TEM、EDS、FT-IR、TGA、N2吸附-脱附、孔径分布等表征了样品的结构和理化性质。XRD和FE-SEM结果证实,以2 wt%的ETS-4种子和3 h的结晶时间合成的复合材料具有ETS-4@LSX结构的纯相。hrtem成像显示LSX在ETS-4表面均匀生长,形成连续的壳层。2 wt% ETS-4@LSX复合材料在1 bar和303 K下的CO2/N2平衡选择性为81.4,分别是纯LSX和ETS-4的5倍和7倍。在20 bar下,该复合材料的CO2吸收量为5.25 mmol g - 1, N2吸收量为0.50 mmol g - 1。动态吸附研究表明,2 wt% ETS-4@LSX的CO2吸附量为3.52 mmol g−1,N2吸附量为0.26 mmol g−1。ETS-4@LSX的增强吸附能力和选择性归因于其双孔结构,突出了其作为从烟气中捕获二氧化碳的有效吸附剂的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


