Enhanced photocatalytic degradation of diclofenac under LED light using Co2SnO4/Co3O4/SnO2: Efficiency, mechanism, and by-product toxicity prediction
IF 4.7
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2025-08-30
DOI:10.1016/j.jphotochem.2025.116742
引用次数: 0
Abstract
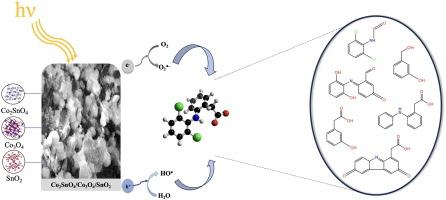
In this study, a Co2SnO4/Co3O4/SnO2 material synthesized via the hydrothermal method was investigated for the degradation of diclofenac (DCF) under LED light. Structural characterization confirmed the presence of spinel and oxide phases with Co2+, Co3+, and Sn4+ oxidation states. Electrochemical analyses indicated efficient charge separation and low interfacial resistance under illumination, highlighting strong photocatalytic potential. A degradation efficiency of 99.45 % and superior kinetic performance, with higher rate constants and shorter half-lives, were achieved via the photocatalytic process compared to 76.25 % in the photocatalytic-PMS system, likely due to the higher reactivity of OH• compared to SO5•- radicals. Scavenger tests revealed that hydroxyl radicals (OH•) were the main reactive species responsible for the degradation of DCF. Additionally, although DCF was completely degraded during the photocatalytic process, the mineralization rate was 39.11 %. Degradation products were identified using LC-ESI-MS, and their predicted toxicity was evaluated using the ECOSAR 2.2 software. A possible degradation pathway was proposed.
Co2SnO4/Co3O4/SnO2增强LED光催化降解双氯芬酸的效率、机理及副产物毒性预测
本研究采用水热法制备Co2SnO4/Co3O4/SnO2材料,研究其在LED光下对双氯芬酸(DCF)的降解作用。结构表征证实了尖晶石和氧化相的存在,氧化态分别为Co2+、Co3+和Sn4+。电化学分析表明,该材料在光照下电荷分离效率高,界面电阻低,具有较强的光催化潜力。与光催化- pms体系的76.25%的降解效率相比,光催化体系的降解效率为99.45%,具有更高的速率常数和更短的半衰期,这可能是由于OH•自由基比SO5•-自由基具有更高的反应活性。清除试验表明,羟基自由基(OH•)是降解DCF的主要活性物质。此外,虽然DCF在光催化过程中被完全降解,但矿化率为39.11%。使用LC-ESI-MS鉴定降解产物,并使用ECOSAR 2.2软件评估其预测毒性。提出一种可能的降解途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


