Biomolecule-tailored chiral nickel oxide nanozymes amplify autophagy-mediated tumor ablation via synergistic photodynamic-chemodynamic therapy
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Chiral nanomaterial-mediated phototherapy has attracted attention as an emerging tumor treatment method. In this study, we synthesized chiral nickel oxide (L/D-Cys-NiO) photosensitizers via surface modulation using chiral cysteine molecules, thereby improving the biocompatibility and significantly enhancing the efficacy of photodynamic-chemodynamic therapy. L/D-Cys-NiO can selectively stimulate the production of reactive oxygen species and hydroxyl radicals, providing synergistic therapy in tumor cells with 4-5-fold greater efficacy than conventional phototherapy. L/D-Cys-NiO can also effectively prevent the infiltrative growth and metastasis of tumor cells by reducing their migration and invasiveness by nearly 40 %. In vivo immunohistochemical assays and high-throughput proteomic analysis confirmed that D-Cys-NiO exhibited stronger enhanced permeability and retention effects and induced more significant autophagic behavior than L-Cys-NiO, suggesting a chirality-dependent enhanced autophagy pathway for tumor ablation during photodynamic-chemodynamic synergistic therapy. We expect that these findings will provide exceptional insights into next-generation tumor photosensitizer designs, focusing on the regulation of autophagy in tumor cells through chirality-dependent photoinduction.
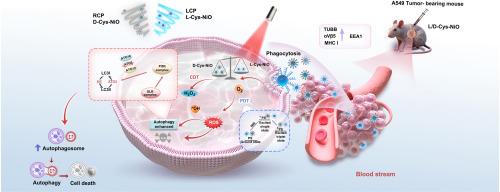
生物分子定制的手性氧化镍纳米酶通过协同光动力-化学动力治疗增强自噬介导的肿瘤消融
手性纳米材料介导的光疗作为一种新兴的肿瘤治疗方法受到了广泛的关注。在本研究中,我们利用手性半胱氨酸分子通过表面调制合成了手性氧化镍(L/D-Cys-NiO)光敏剂,从而改善了生物相容性,显著提高了光动力-化学动力治疗的疗效。L/D-Cys-NiO可以选择性刺激活性氧和羟基自由基的产生,为肿瘤细胞提供协同治疗,其疗效比传统光疗提高4-5倍。L/D-Cys-NiO还能有效阻止肿瘤细胞的浸润性生长和转移,使肿瘤细胞的迁移性和侵袭性降低近40%。体内免疫组织化学分析和高通量蛋白质组学分析证实,D-Cys-NiO比L-Cys-NiO具有更强的增强渗透性和保留作用,诱导了更显著的自噬行为,提示光动力-化学动力协同治疗中肿瘤消融的增强自噬途径依赖于手性。我们期望这些发现将为下一代肿瘤光敏剂的设计提供独特的见解,重点是通过手性依赖的光诱导来调节肿瘤细胞的自噬。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


