A viscosity-activable nano-micelle with aggregation-induced emission for boosting NIR-II fluorescence imaging-guided synergetic photodynamic/photothermal therapy of breast cancer
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
In view of the fast-growing achievements of cancer phototheranostics, the exploration of advanced materials shows inexhaustible and vigorous vitality. To overcome those limitations of existing materials, such as aggregation-caused fluorescence quenching, inferior selectivity, and high O2-dependence, the utilization of specific activatable agents with aggregation-induced emission (AIE) features is highly desirable yet remains challenging. Herein, we have designed an organic small-molecule microenvironment activated AIE agent, namely DPXBI. After encapsulation into amphiphilic DSPE-PEG-RGD through self-assembly, the biocompatibility, water solubility, targeting effect, and circulation time in the blood of obtained nano-micelle RM@DPXBI are improved. RM@DPXBI showed powerful fluorescence in the second near-infrared (NIR-II) region after being activated by viscosity in the tumor microenvironment, which could accurately identify the boundary of the tumor and determine the optimal accumulation time for following phototherapy. Subsequently, RM@DPXBI exerts inhibiting on breast cancer growth through synergistic photodynamic (PDT) and photothermal (PTT) therapy under single laser irradiation. Meanwhile, the phototherapy-induced cellular death increases intracellular viscosity, which further restricts the intramolecular motion of RM@DPXBI, resulting in the amplifying of PDT effect. The activated strategy provides a new exploration for the design and development of AIE and viscosity activation agents in tumor theranostics.
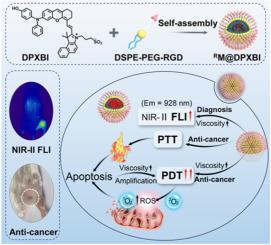
具有聚集诱导发射的黏度可激活纳米胶束,用于促进NIR-II荧光成像引导的乳腺癌协同光动力/光热治疗
鉴于癌症光疗的快速发展成果,对先进材料的探索显示出取之不尽、旺盛的生命力。为了克服现有材料的局限性,如聚集引起的荧光猝灭、低选择性和对o2的高依赖性,利用具有聚集诱导发射(AIE)特征的特异性活化剂是非常可取的,但仍然具有挑战性。为此,我们设计了一种有机小分子微环境激活AIE剂DPXBI。通过自组装将纳米胶束RM@DPXBI包封在两亲性的DSPE-PEG-RGD中,提高了其生物相容性、水溶性、靶向性和血液循环时间。RM@DPXBI在肿瘤微环境中被黏度激活后,在第二近红外区(NIR-II)表现出强大的荧光,可以准确识别肿瘤边界,确定后续光治疗的最佳积累时间。随后,RM@DPXBI通过单次激光照射下的光动力(PDT)和光热(PTT)协同治疗对乳腺癌的生长产生抑制作用。同时,光疗诱导的细胞死亡增加了细胞内黏度,进一步限制了RM@DPXBI的分子内运动,导致PDT效应放大。激活策略为肿瘤治疗中AIE和黏度活化剂的设计和开发提供了新的探索。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


