Engineering cation vacancy defects on LaNiO3 for efficient oxygen evolution reaction
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
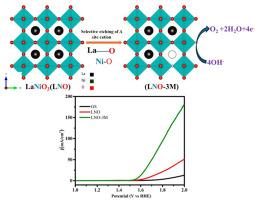
Perovskite oxides (ABO3) have been extensively studied as electrocatalysts for the oxygen evolution reaction (OER) due to their highly tunable and flexible electronic structures. However, their practical use as electrocatalysts has been hindered by limitations such as less surface area and low electrical conductivity. In this work LaNiO3 (LNO) perovskite oxides were synthesized and modified by selectively removing A-site cations by etching with dilute acetic acid treatment. This acid etching process induced cation and oxygen vacancies, resulting in an increased surface area, and enhanced surface wettability, facilitating improved interaction with water molecules thereby promoting a higher density of catalytically active sites. These structural and surface modifications led to superior OER performance in alkaline media. The optimized catalyst, LNO-3M, demonstrated a reduced overpotential, smaller Tafel slope, higher turnover frequency, and greater mass activity compared to pristine LNO. Electrochemical measurements confirmed faster charge transfer and a higher density of active sites due to the increased electrochemical surface area. Furthermore, LNO-3M maintained excellent operational stability over extended periods. This study underscores the efficacy of a simple acid etching strategy to enhance the catalytic activity of LaNiO3 for efficient water splitting applications.
用于高效析氧反应的工程阳离子空位缺陷
钙钛矿氧化物(ABO3)由于其高度可调和灵活的电子结构而被广泛研究作为析氧反应(OER)的电催化剂。然而,它们作为电催化剂的实际应用受到诸如表面积小和导电性低等限制。本文采用稀乙酸蚀刻法选择性去除a位阳离子的方法合成了LaNiO3 (LNO)钙钛矿氧化物。这种酸蚀过程诱导阳离子和氧空位,导致表面积增加,表面润湿性增强,促进与水分子的相互作用,从而促进更高密度的催化活性位点。这些结构和表面修饰导致了在碱性介质中优异的OER性能。与原始LNO相比,优化后的LNO- 3m催化剂的过电位降低,Tafel斜率较小,周转频率更高,质量活性更高。电化学测量证实,由于电化学表面积的增加,电荷转移更快,活性位点密度更高。此外,LNO-3M在较长时间内保持了良好的运行稳定性。这项研究强调了一种简单的酸蚀刻策略对提高LaNiO3的催化活性的有效性,用于高效的水裂解应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


