Effect of high-temperature treatment with orthophosphoric acid on the phase composition, elemental composition, microstructure and electrical properties of calcium sulfate hemihydrate
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Citrogypsum, which is waste product of biochemical production of citric acid, has been applied to prepare bulk samples of calcium sulfate hemihydrate, CaSO4∙0.5H2O. The samples were undergone to high-temperature (95 °C) treatment in 85 wt% aqueous solution of orthophosphoric acid at different treatment times, th (th = 1; 3; 5; 7; 10 and 15 min). Due to interaction between CaSO4∙0.5H2O and H2O from aqueous solution, at first treatment stage, the starting calcium sulfate hemihydrate quickly (at th < 1 min) and completely transforms into calcium sulfate dihydrate, CaSO4∙2H2O. With further increase in th, phase composition of the samples changes from single-phased state with monoclinic CaSO4∙2H2O structure at th = 1min to two-phased state at th > 1 min, in which new phase of calcium sulfate hemihydrate CaSO4∙0.5H2O with pseudo-trigonal structure is gradually forming from phase CaSO4∙2H2O. Formation of two-phased state is related to phase transformation, originated from partial removal of crystallization water molecules from the CaSO4·2H2O structure. During the acid treatment, in the samples content of Ca and S decrease, and content of P increases. These changes in elemental composition are governed by mechanisms of cation exchange (via partial replacement of Ca2+ cation by two H+ ions) and anion exchange (via partial replacement of acid group (SO4)2- by acid residue (HPO4)2-). Electrical properties of the samples were examined by analyzing of room-temperature voltage-current dependencies, recording at 50 Hz. Electrical conductivity of the samples are gradually increasing with increasing th. This effect can be related to appearance of mobile H+ ions in structure of the samples. Highest value of the conductivity is ∼0.058 S∙m−1 for th = 15 min. Increase in the conductivity is accompanied by gradual increase in relaxational current contribution into total electric current. This feature can be originated from formation of acid residues (PO4)3-, which partially replace acid residue (SO4)2- in the CaSO4 structure of the acid treated samples.
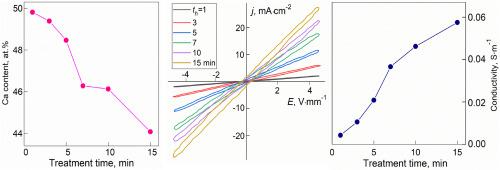
正磷酸高温处理对半水合硫酸钙相组成、元素组成、微观结构和电性能的影响
利用柠檬酸生化生产的废渣——柠檬石膏制备了半水硫酸钙CaSO4∙0.5H2O散装样品。样品在85 wt%的正磷酸水溶液中进行95℃高温处理,处理时间分别为1、3、5、7、10、15 min。由于CaSO4∙0.5H2O与水溶液中的H2O相互作用,在第一处理阶段,起始硫酸钙半水合反应迅速(1 min),并完全转化为二水合硫酸钙CaSO4∙2H2O。随着时间的进一步增加,样品的相组成在1min时由单斜CaSO4∙2H2O的单相结构转变为1min时的两相结构,其中CaSO4∙2H2O相逐渐形成半水硫酸钙CaSO4∙0.5H2O的拟三角结构新相。两相态的形成与相变有关,源于CaSO4·2H2O结构中结晶水分子的部分去除。酸处理过程中,样品中Ca、S含量降低,P含量升高。这些元素组成的变化是由阳离子交换(通过两个H+离子部分取代Ca2+阳离子)和阴离子交换(通过酸渣(HPO4)2-部分取代酸基(SO4)2-)的机制控制的。通过分析室温电压-电流依赖关系来检测样品的电学性能,并在50 Hz下记录。样品的电导率随温度的增加而逐渐增大。这种效应可能与样品结构中流动氢离子的出现有关。在15 min内,电导率的最大值为~ 0.058 S∙m−1。电导率的增加伴随着松弛电流对总电流的贡献的逐渐增加。这种特征可能源于酸渣(PO4)3-的形成,它部分取代了酸渣(SO4)2-在酸处理样品的CaSO4结构中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


