Ion-selective change storage in three types layered MnO2 films intercalated with different cobalt complexes
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
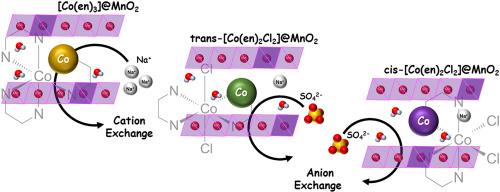
In this study, layered MnO2 films intercalated with three types of cobalt complexes; [Co(en)3], trans-[Co(en)2Cl2], and cis-[Co(en)2Cl2] were prepared on FTO substrates, forming [Co(en)3]@MnO2, trans-[Co(en)2Cl2]@MnO2, and cis-[Co(en)2Cl2]@MnO2, respectively. XRD, EDS, FT-IR, and Raman results confirmed the preparation of layered MnO2 films with the respective cobalt complexes in the interlayer. Electrochemical measurements (cyclic voltammetry; CV) in Na2SO4 electrolyte showed that [Co(en)3]@MnO2 film (262 F/g), trans-[Co(en)2Cl2]@MnO2 film (226 F/g) and cis-[Co(en)2Cl2]@MnO2 film (193 F/g), showing high cycle stability and capacitance. During CV measurements, the cobalt complex in [Co(en)3]@MnO2 was deintercalated and replaced by hydrated Na + ions, whereas in trans-[Co(en)2Cl2]@MnO2 and cis-[Co(en)2Cl2]@MnO2, the cobalt complexes retained in the interlayer. These results suggest that Na+ ions intercalation/deintercalation dominated in [Co(en)3]@MnO2, while SO42− ions intercalation/deintercalation occurred in trans-[Co(en)2Cl2]@MnO2 and cis-[Co(en)2Cl2]@MnO2. The ionic property that Na + ions diffuse more easily in aqueous solution than SO42− ions suggests that the [Co(en)3]@MnO2 film has a larger capacitance than the trans-[Co(en)2Cl2]@MnO2 film and the cis-[Co(en)2Cl2]@MnO2 film. Electrochemical impedance spectroscopy (EIS), [Co(en)3]@MnO2 exhibited a mixed response of capacitive and diffusive behavior associated with Na + replacement, with a linear region showing a slope of approximately 60° in the Nyquist plot. trans-[Co(en)2Cl2]@MnO2 and cis-[Co(en)2Cl2]@MnO2 showed two semicircles originating from surface and interlayer reactions, followed by a linear region with a slope of about 80°, reflecting surface-capacitive dominance. These features are consistent with the differences in the slope and phase angle observed in the Bode plots, indicating that the EIS response is influenced by ion diffusivity and interlayer structure.
三种不同钴配合物层状二氧化锰膜的离子选择性变化存储
在本研究中,层状MnO2膜嵌入了三种类型的钴配合物;在FTO衬底上制备了[Co(en)3]、trans-[Co(en)2Cl2]和顺式-[Co(en)2Cl2],分别形成了[Co(en)3]@MnO2、trans-[Co(en)2Cl2]@MnO2和顺式-[Co(en)2Cl2]@MnO2。XRD, EDS, FT-IR和Raman结果证实了层状MnO2薄膜的制备,层间分别有钴配合物。在Na2SO4电解质中的电化学测试(循环伏安法;CV)表明,[Co(en)3]@MnO2膜(262 F/g)、反式-[Co(en)2Cl2]@MnO2膜(226 F/g)和顺式-[Co(en)2Cl2]@MnO2膜(193 F/g)表现出较高的循环稳定性和电容。在CV测量中,[Co(en)3]@MnO2中的钴配合物被脱嵌并被水合的Na +离子取代,而在反式-[Co(en)2Cl2]@MnO2和顺式-[Co(en)2Cl2]@MnO2中,钴配合物保留在中间层中。结果表明,在[Co(en)3]@MnO2中,Na+离子的插入/脱嵌作用占主导地位,而在反式-[Co(en)2Cl2]@MnO2和顺式-[Co(en)2Cl2]@MnO2中,SO42−离子的插入/脱嵌作用占主导地位。Na +离子比SO42−离子更容易在水溶液中扩散,表明[Co(en)3]@MnO2薄膜比反式-[Co(en)2Cl2]@MnO2薄膜和顺式-[Co(en)2Cl2]@MnO2薄膜具有更大的电容。电化学阻抗谱(EIS)显示,[Co(en)3]@MnO2在Na +置换过程中表现出电容性和扩散性的混合响应,在Nyquist图中线性区呈现出约60°的斜率。反式-[Co(en)2Cl2]@MnO2和顺式-[Co(en)2Cl2]@MnO2表现出两个源自表面和层间反应的半圆,然后是一个斜率约为80°的线性区域,反映了表面电容性优势。这些特征与Bode图中观察到的斜率和相角的差异一致,表明EIS响应受离子扩散率和层间结构的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


