The ameliorative effects of Honokiol on intracerebroventricular Kainic acid-induced spinal cord neurotoxicity
IF 2.4
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
Kainic acid (Ka) is a neuroexcitatory agent commonly utilized in the modeling of excitotoxicity. The study aims to investigate both the neurotoxic effects of intracerebroventricular (icv) Ka injections in rats on the cervical spinal cord and the therapeutic role of honokiol. Fifty male Wistar Albino rats (n = 10) are divided into five groups. The control group of rats received normal saline by intraperitoneal (ip) injection for a duration of 7 days. The sham group of rats was administered a single dose of icv normal saline on the first day. Rats in the Ka group received a single icv dosage of Ka (0.5 μg/μl). Honokiol (Hnl) group rats Hnl for 7 days (ip-5 mg/kg), while Ka + Hnl group rats were administered Ka (single dose) and Hnl (7 days). Exhibited substantial histopathological alterations and an increase in glial fibrillary acidic protein (GFAP) positive (+) cells in the rats in the Ka group. In the Ka + Hnl group of rats, an improvement in histological structure and a reduction in GFAP (+) cells compared to the Ka group. The central canal lumen diameters were significantly expanded in the Ka group rats. It was observed that malondialdehyde (MDA) and tumor necrosis factor-α (TNF-α) levels increased and glutathione (GSH) levels decreased in the spinal cord of rats in the Ka group. Hnl treatment caused a significant improvement in MDA and GSH levels and a decrease in TNF-α levels in rats with the excitotoxicity model. In conclusion, the findings showed that Hnl treatment attenuated Ka-induced neurotoxicity by reducing oxidative tissue damage, inflammatory response, and GFAP expression.
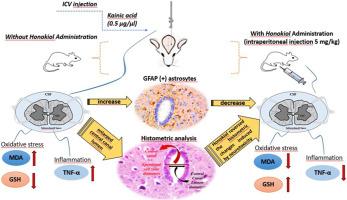
厚朴酚对脑室内凯尼克酸所致脊髓神经毒性的改善作用
Kainic acid (Ka)是一种神经兴奋剂,通常用于兴奋性毒性的建模。本研究旨在探讨大鼠脑室注射Ka对颈脊髓的神经毒性作用及本木酚的治疗作用。50只雄性Wistar Albino大鼠(n = 10)分为5组。对照组大鼠腹腔注射生理盐水,持续7 d。假手术组大鼠第1天给予单剂量生理盐水。Ka组大鼠给予单icv剂量的Ka (0.5 μg/μl)。本木酚(Hnl)组大鼠给药Hnl 7 d (ip-5 mg/kg), Ka + Hnl组大鼠给药Ka(单剂量)和Hnl (7 d)。Ka组大鼠表现出明显的组织病理学改变,胶质纤维酸性蛋白(GFAP)阳性(+)细胞增多。与Ka组相比,Ka + Hnl组大鼠组织学结构改善,GFAP(+)细胞减少。Ka组大鼠中央管腔直径明显扩大。结果显示,Ka组大鼠脊髓内丙二醛(MDA)和肿瘤坏死因子-α (TNF-α)水平升高,谷胱甘肽(GSH)水平降低。Hnl处理引起兴奋性毒性模型大鼠MDA和GSH水平的显著改善和TNF-α水平的降低。总之,研究结果表明,Hnl治疗通过减少氧化组织损伤、炎症反应和GFAP表达来减轻ka诱导的神经毒性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Toxicon
医学-毒理学
CiteScore
4.80
自引率
10.70%
发文量
358
审稿时长
68 days
期刊介绍:
Toxicon has an open access mirror Toxicon: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review. An introductory offer Toxicon: X - full waiver of the Open Access fee.
Toxicon''s "aims and scope" are to publish:
-articles containing the results of original research on problems related to toxins derived from animals, plants and microorganisms
-papers on novel findings related to the chemical, pharmacological, toxicological, and immunological properties of natural toxins
-molecular biological studies of toxins and other genes from poisonous and venomous organisms that advance understanding of the role or function of toxins
-clinical observations on poisoning and envenoming where a new therapeutic principle has been proposed or a decidedly superior clinical result has been obtained.
-material on the use of toxins as tools in studying biological processes and material on subjects related to venom and antivenom problems.
-articles on the translational application of toxins, for example as drugs and insecticides
-epidemiological studies on envenoming or poisoning, so long as they highlight a previously unrecognised medical problem or provide insight into the prevention or medical treatment of envenoming or poisoning. Retrospective surveys of hospital records, especially those lacking species identification, will not be considered for publication. Properly designed prospective community-based surveys are strongly encouraged.
-articles describing well-known activities of venoms, such as antibacterial, anticancer, and analgesic activities of arachnid venoms, without any attempt to define the mechanism of action or purify the active component, will not be considered for publication in Toxicon.
-review articles on problems related to toxinology.
To encourage the exchange of ideas, sections of the journal may be devoted to Short Communications, Letters to the Editor and activities of the affiliated societies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


