GPR91 mediates low shear stress-induced endothelial ferroptosis via EGR1-dependent GPX4 repression
IF 3.7
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Emerging evidence suggests that low shear stress (LSS) contributes to endothelial injury in atherosclerosis, yet the underlying molecular mechanisms remain incompletely understood. Here, we demonstrate that LSS triggers ferroptosis in vascular endothelial cells through a novel GPR91/EGR1/GPX4 signaling axis. By integrating bioinformatics, single-cell RNA sequencing (scRNA-seq), and ChIP-qPCR, we identified a significant correlation between LSS-responsive genes and ferroptosis-related pathways in atherosclerotic plaques. Using parallel-plate flow chamber system, we confirmed that LSS (3 dyne/cm2) induces characteristic ferroptosis markers in human vascular endothelial cells. Mechanistically, LSS upregulated GPR91, enhancing cellular mechanosensitivity, as further validated in HEK293T cells. Pharmacological inhibition of GPR91 attenuated LSS-induced ferroptosis, while its agonists exacerbated ferroptosis. RNA-seq and ChIP-qPCR identified EGR1 as a downstream of GPR91 effector that binds directly to the GPX4 promoter (M2 motif), repressing its transcription under LSS. Our findings establish GPR91 as a mechanosensitive receptor that links LSS to ferroptosis signaling via EGR1- mediated GPX4 repression, providing new therapeutic targets for shear stress-related vascular pathologies.
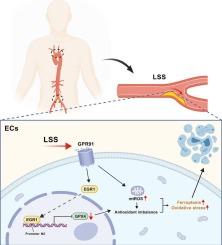
GPR91通过egr1依赖的GPX4抑制介导低剪切应力诱导的内皮细胞铁下垂
新出现的证据表明,低剪切应力(LSS)有助于动脉粥样硬化中的内皮损伤,但其潜在的分子机制仍不完全清楚。在这里,我们证明LSS通过一个新的GPR91/EGR1/GPX4信号轴触发血管内皮细胞的铁下垂。通过整合生物信息学、单细胞RNA测序(scRNA-seq)和ChIP-qPCR,我们发现了动脉粥样硬化斑块中lss应答基因与铁凋亡相关途径之间的显著相关性。利用平行板流室系统,我们证实了LSS (3 dyne/cm2)在人血管内皮细胞中诱导了特征性的铁下垂标志物。在机制上,LSS上调GPR91,增强细胞的机械敏感性,这在HEK293T细胞中得到进一步验证。药理抑制GPR91可减轻lss诱导的铁下垂,而其激动剂可加重铁下垂。RNA-seq和ChIP-qPCR鉴定EGR1是GPR91效应子的下游,直接结合GPX4启动子(M2基序),在LSS下抑制其转录。我们的研究结果证实GPR91是一种机械敏感受体,通过EGR1介导的GPX4抑制将LSS与铁死亡信号联系起来,为剪切应力相关血管病变提供了新的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


