W1/O/W2 double emulsions stabilized by natural diosgenin and ultrasound-treated pea protein nanoparticles: A plant-based system for iron fortification
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
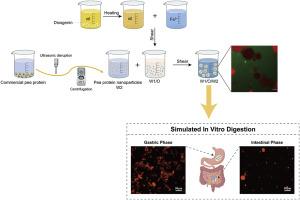
This study developed and optimized a fully natural W1/O/W2 double emulsion system for oral iron delivery, using diosgenin, a natural triterpenoid saponin, to stabilize the inner water–oil (W1/O) interface and pea protein nanoparticles (PPNs) to stabilize the outer oil–water (O/W2) interface. The optimal primary emulsion, containing 9 % diosgenin in the oil phase and a W1:O ratio of 2:8, exhibited excellent centrifugal and storage stability. External aqueous phases (W2) were prepared from 2 %, 4 %, and 6 % pea protein dispersions, which underwent ultrasound treatment followed by centrifugation to produce corresponding PPNs suspensions designated as PPN-2 %, PPN-4 %, and PPN-6 %, respectively. The primary iron-containing W1/O emulsion was dispersed into these W2 phases at a W1/O: W2 ratio of 2:8, yielding stable W1/O/W2 emulsions with iron encapsulation efficiencies exceeding 99 %. In vitro simulated digestion revealed that the emulsions with PPN-6 % effectively minimized iron release in the gastric phase (<1 %) while promoting controlled release in the intestinal phase, thereby improving bioavailability and potentially reducing gastric irritation. Compared to synthetic PGPR-stabilized emulsions, the diosgenin-PPNs system demonstrated superior structural stability and digestive performance. This multilayered, food-grade emulsion system offers a promising, health-oriented alternative for iron fortification in functional foods.
由天然薯蓣皂苷元和超声波处理的豌豆蛋白纳米颗粒稳定的W1/O/W2双乳:一种基于植物的铁强化系统
本研究利用天然三萜皂苷薯蓣皂苷元稳定内层水-油(W1/O)界面,利用豌豆蛋白纳米颗粒(PPNs)稳定外层油-水(O/W2)界面,开发并优化了一种全天然口服铁离子双乳体系。优选的初乳油相中薯蓣皂苷元含量为9%,W1:O比为2:8,具有良好的离心稳定性和贮存稳定性。用2%、4%和6%的豌豆蛋白分散体制备外水相(W2),超声处理后离心得到相应的ppn悬浮液,分别为ppn - 2%、ppn - 4%和ppn - 6%。以W1/O: W2为2:8的比例将含铁原浆W1/O/W2分散到这些W2相中,得到稳定的W1/O/W2乳液,铁包封率超过99%。体外模拟消化实验表明,ppn - 6%乳状液能有效减少胃相铁释放(1%),同时促进肠相铁的控释,从而提高生物利用度,并有可能减少胃刺激。与合成的pgpr稳定乳剂相比,薯蓣皂苷元- ppns体系具有更好的结构稳定性和消化性能。这种多层次的食品级乳剂系统为功能性食品中的铁强化提供了一种有前途的、以健康为导向的选择。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


