Silver nanoparticles amplify mercury toxicity in rice: Impacts on germination, growth, and cellular integrity
IF 5.7
2区 生物学
Q1 PLANT SCIENCES
引用次数: 0
Abstract
This study investigates the synergistic co-effects of mercury (Hg) and silver nanoparticles (AgNPs) on rice seed germination and early seedling development. Hg were applied at concentrations of 0, 0.33, 1.65, and 8.25 mg kg−1 while AgNPs were administered at 0, 50, 100, and 500 mg kg−1. Results demonstrate that single low Hg concentrations enhanced germination rate from 79.63 % to 89.51 %, and stimulated α-amylase activity from 0.26 to 0.34 μg g−1 FW min−1. Co-exposure with AgNPs significantly modulated these responses, particularly under 50 and 500 mg kg−1 AgNPs treatments where α-amylase activity reached from 0.26 to 0.49 and 0.25 μg g−1 FW min−1, respectively. The combined treatment with high concentrations of both contaminants further substantially inhibited seedling growth, reducing plant height by 39 % and roots length by 13 %. AgNPs further enhanced Hg accumulation in plant tissues and intensified oxidative stress, as indicated by elevated peroxidase (POD) activity and malondialdehyde (MDA) levels. Ultrastructural and DNA damage analyses confirmed severe cellular disruption and dose-dependent genotoxicity. These findings provide critical insights into the synergistic toxicity mechanisms of Hg and AgNPs co-contamination in rice seedlings, with important implications for agricultural safety under heavy metal and nano-product combined contamination.
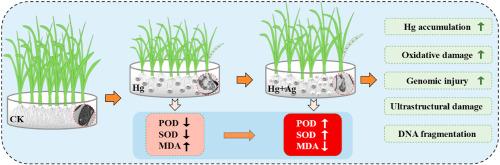
银纳米粒子增强汞对水稻的毒性:对萌发、生长和细胞完整性的影响
本文研究了纳米汞(Hg)和纳米银(AgNPs)对水稻种子萌发和幼苗早期发育的协同效应。Hg的浓度分别为0、0.33、1.65和8.25 mg kg - 1, AgNPs的浓度分别为0、50、100和500 mg kg - 1。结果表明,单次低汞浓度可使种子发芽率从79.63%提高到89.51%,α-淀粉酶活性从0.26提高到0.34 μg−1 FW min−1。与AgNPs共暴露可显著调节这些反应,特别是在50和500 mg kg - 1 AgNPs处理下,α-淀粉酶活性分别达到0.26 ~ 0.49和0.25 μg - 1 FW min - 1。高浓度两种污染物的联合处理进一步显著抑制了幼苗的生长,使株高减少39%,根长减少13%。AgNPs通过提高过氧化物酶(POD)活性和丙二醛(MDA)水平,进一步促进了汞在植物组织中的积累,并加剧了氧化胁迫。超微结构和DNA损伤分析证实了严重的细胞破坏和剂量依赖性遗传毒性。这些发现为汞和AgNPs共同污染水稻幼苗的协同毒性机制提供了重要见解,对重金属和纳米产品联合污染下的农业安全具有重要意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.10
自引率
3.10%
发文量
410
审稿时长
33 days
期刊介绍:
Plant Physiology and Biochemistry publishes original theoretical, experimental and technical contributions in the various fields of plant physiology (biochemistry, physiology, structure, genetics, plant-microbe interactions, etc.) at diverse levels of integration (molecular, subcellular, cellular, organ, whole plant, environmental). Opinions expressed in the journal are the sole responsibility of the authors and publication does not imply the editors'' agreement.
Manuscripts describing molecular-genetic and/or gene expression data that are not integrated with biochemical analysis and/or actual measurements of plant physiological processes are not suitable for PPB. Also "Omics" studies (transcriptomics, proteomics, metabolomics, etc.) reporting descriptive analysis without an element of functional validation assays, will not be considered. Similarly, applied agronomic or phytochemical studies that generate no new, fundamental insights in plant physiological and/or biochemical processes are not suitable for publication in PPB.
Plant Physiology and Biochemistry publishes several types of articles: Reviews, Papers and Short Papers. Articles for Reviews are either invited by the editor or proposed by the authors for the editor''s prior agreement. Reviews should not exceed 40 typewritten pages and Short Papers no more than approximately 8 typewritten pages. The fundamental character of Plant Physiology and Biochemistry remains that of a journal for original results.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


