Hypoxia-responsive Exatecan prodrug liposomes co-delivered with IR808 for synergistic chemo–photodynamic therapy of breast cancer
IF 6.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
To overcome the poor solubility, rapid clearance, and systemic toxicity of Exatecan, we developed a tumor-activated liposomal delivery system co-encapsulating a hypoxia-cleavable Exatecan–squalene prodrug (EXA PRO) and the photosensitizer IR808. The nanocarrier integrates photodynamic therapy-induced hypoxia with bioreductive drug activation, enabling spatiotemporally controlled release within the tumor microenvironment. The liposomes, formulated with pH-sensitive lipids, exhibited uniform size (∼136 nm), high encapsulation efficiency, and dual responsiveness to acidic pH and reductive stress. Upon near-infrared laser irradiation, IR808 generated reactive oxygen species and aggravated intratumoral hypoxia, thereby triggering the cleavage of the azobenzene linker and facilitating targeted release of Exatecan. In vitro and in vivo studies confirmed enhanced cellular uptake, efficient intracellular drug release, potent tumor growth inhibition, and favorable biosafety profiles. This synergistic chemo–photodynamic strategy offers a promising platform for precise, tumor-selective drug delivery and represents a potential advancement in personalized cancer therapy.
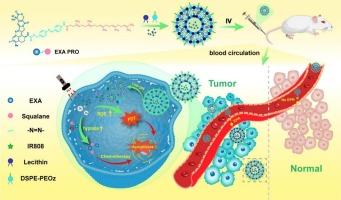
缺氧反应的Exatecan前药脂质体与IR808共同递送用于乳腺癌的协同化学光动力治疗
为了克服Exatecan的低溶解度、快速清除和全身性毒性,我们开发了一种肿瘤激活的脂质体递送系统,共包被缺氧可切割的Exatecan -角鲨烯前药(EXA PRO)和光敏剂IR808。该纳米载体将光动力疗法诱导的缺氧与生物还原性药物激活结合在一起,实现了肿瘤微环境内的时空可控释放。脂质体由pH敏感脂质配制而成,具有均匀大小(~ 136 nm)、高包封效率和对酸性pH和还原胁迫的双重响应性。在近红外激光照射下,IR808产生活性氧,加重肿瘤内缺氧,从而触发偶氮苯连接物的裂解,促进Exatecan的靶向释放。体外和体内研究证实了增强细胞摄取,有效的细胞内药物释放,有效的肿瘤生长抑制和良好的生物安全性。这种协同化学光动力学策略为精确的肿瘤选择性药物递送提供了一个有前途的平台,并代表了个性化癌症治疗的潜在进步。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Pharmaceutics: X
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
6.60
自引率
0.00%
发文量
32
审稿时长
24 days
期刊介绍:
International Journal of Pharmaceutics: X offers authors with high-quality research who want to publish in a gold open access journal the opportunity to make their work immediately, permanently, and freely accessible.
International Journal of Pharmaceutics: X authors will pay an article publishing charge (APC), have a choice of license options, and retain copyright. Please check the APC here. The journal is indexed in SCOPUS, PUBMED, PMC and DOAJ.
The International Journal of Pharmaceutics is the second most cited journal in the "Pharmacy & Pharmacology" category out of 358 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


