Fc-tagged PLA2R antigen enables high-performance chemiluminescent detection for non-invasive idiopathic membranous nephropathy diagnosis
IF 3.7
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
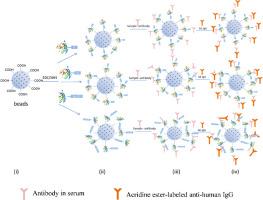
Idiopathic membranous nephropathy (IMN) is characterized by anti-phospholipase A2 receptor (PLA2R) antibodies, yet current detection methods lack sensitivity or are invasive. We developed a chemiluminescent assay to address these limitations. Three PLA2R antigens (Fc-tagged full, His-tagged, D3 core segment) were expressed in Expi293E cells and purified. Goat/mouse anti-human IgG antibodies were compared for detection efficiency. Kit stability was evaluated at 4 °C and 37 °C. Clinical validation included 75 biopsy-confirmed cases (50 IMN, 25 non-IMN), comparing the kit’s performance against a commercial ELISA. The PLA2R-Fc antigen demonstrated 100 % sensitivity and specificity, outperforming His-tagged (100 % sensitivity, 97 % specificity) and D3-truncated variants (60 % sensitivity). Coating in pH 6.0 MES buffer achieved the highest signal-to-noise ratio (45.08 vs. 25.76 in HEPES). Goat anti-human IgG enabled lower detection limits (22.5 U mL−1 vs. 45 U mL−1 for monoclonal antibody). The kit retained stable over 12 months at 4 °C. In clinical testing, the chemiluminescent assay showed superior concordance with histopathology, higher sensitivity, and specificity, particularly for low-titer samples. This chemiluminescent anti-PLA2R antibody assay combines high sensitivity, specificity, and stability, enabling non-invasive IMN diagnosis and therapeutic monitoring. Its technical optimizations address key limitations of existing methods, offering a reliable diagnostic tool for IMN.
fc标记的PLA2R抗原可用于非侵袭性特发性膜性肾病的高效化学发光检测
特发性膜性肾病(IMN)以抗磷脂酶A2受体(PLA2R)抗体为特征,但目前的检测方法缺乏敏感性或具有侵入性。我们开发了一种化学发光试验来解决这些局限性。三种PLA2R抗原(fc标记full, his标记,D3核心段)在Expi293E细胞中表达并纯化。比较山羊/小鼠抗人IgG抗体的检测效率。在4°C和37°C时评估试剂盒的稳定性。临床验证包括75例活检确诊病例(50例IMN, 25例非IMN),将试剂盒的性能与商业ELISA进行比较。PLA2R-Fc抗原表现出100%的敏感性和特异性,优于his标记的(100%敏感性,97%特异性)和d3截断的变体(60%敏感性)。在pH 6.0的MES缓冲液中涂层获得了最高的信噪比(45.08 vs. 25.76 HEPES)。山羊抗人IgG的检出限较低(单克隆抗体为22.5 U mL−1,单克隆抗体为45 U mL−1)。试剂盒在4°C下保持稳定超过12个月。在临床测试中,化学发光法显示出与组织病理学的优越一致性,更高的灵敏度和特异性,特别是对低滴度样品。这种化学发光抗pla2r抗体检测结合了高灵敏度、特异性和稳定性,使非侵入性IMN诊断和治疗监测成为可能。其技术优化解决了现有方法的主要局限性,为IMN提供了可靠的诊断工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


