RNA binding protein DDX3X drives pancreatic cancer progression via the TLE2-MYL9 axis
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Current treatments for pancreatic ductal adenocarcinoma (PDAC) fall short of meeting clinical needs, highlighting the urgent need for a comprehensive understanding of PDAC progression, which involves not only biochemical signals but also essential biomechanical cues. Here, we used a CRISPR-Cas9 screen in an orthotopic xenograft model to explore PDAC dynamics. The RNA binding protein DEAD-box helicase 3X-linked (DDX3X) was identified as a pivotal oncogene and biomechanical checkpoint. Specifically, DDX3X up-regulation in PDAC promoted tumorigenesis and metastasis, primarily through the transcriptional repressor TLE family member 2 (TLE2). Dysregulation of DDX3X in the tumor destabilized TLE2 messenger RNA and therefore disrupted the interaction with KLF4 (KLF transcription factor 4), leading to increased expression of myosin light chain 9 (MYL9). This change remodeled F-actin, enhancing tumor cell traction forces and consequently facilitating tumor metastasis. Targeting the DDX3X-TLE2-MYL9 pathway considerably reduces PDAC progression. This research reveals a promising approach for treating PDAC by focusing on biomechanical cues.
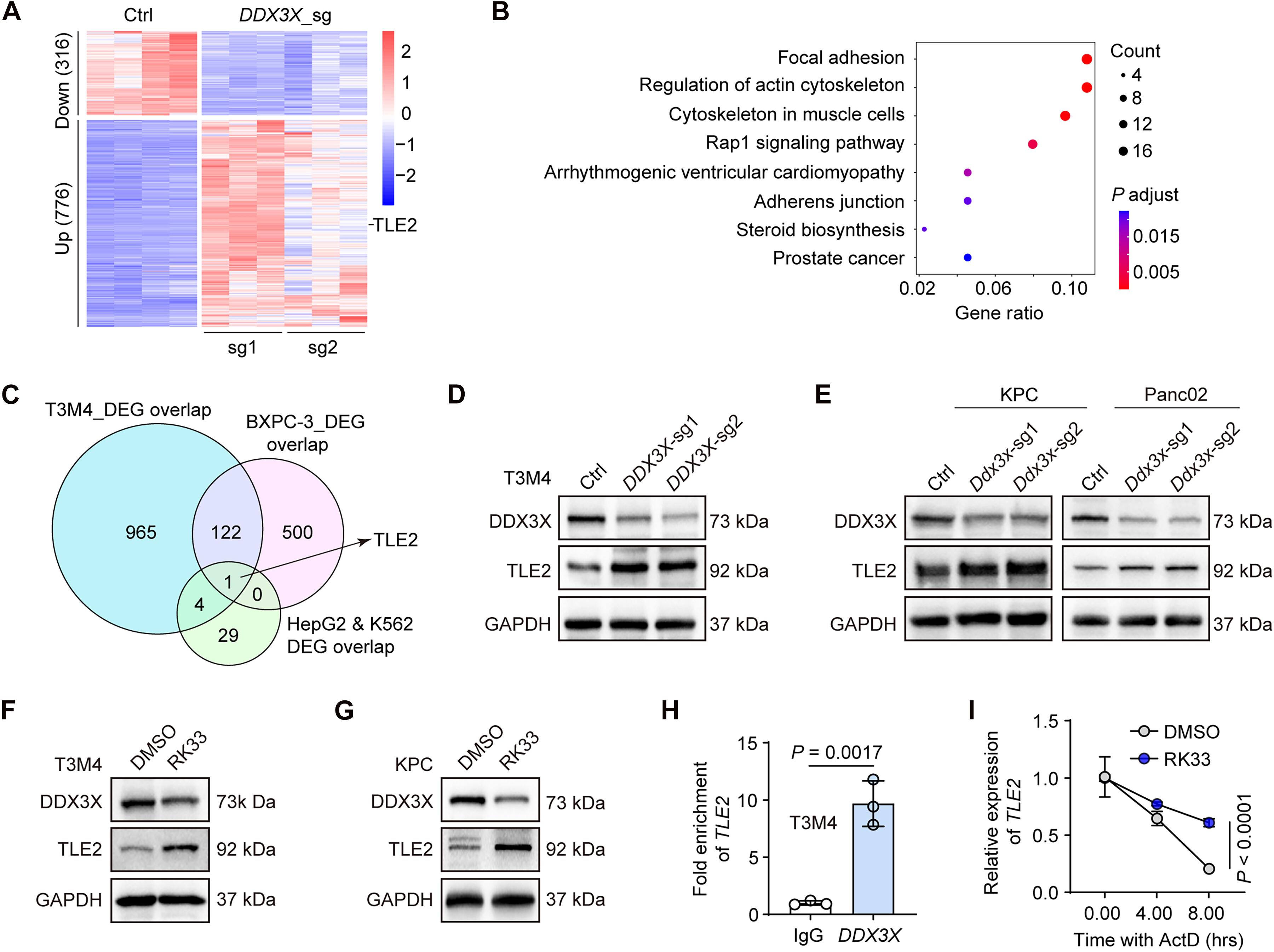
RNA结合蛋白DDX3X通过TLE2-MYL9轴驱动胰腺癌进展。
目前胰腺导管腺癌(pancreatic ductal adencarcinoma, PDAC)的治疗方法不能满足临床需要,迫切需要全面了解PDAC的进展,这不仅包括生化信号,还包括必要的生物力学线索。在这里,我们在一个原位异种移植模型中使用CRISPR-Cas9筛选来探索PDAC动力学。RNA结合蛋白DEAD-box解旋酶3X-linked (DDX3X)被确定为关键的癌基因和生物力学检查点。具体来说,PDAC中DDX3X的上调主要通过转录抑制因子TLE家族成员2 (TLE2)促进肿瘤发生和转移。肿瘤中DDX3X的失调破坏了TLE2信使RNA的稳定,从而破坏了与KLF4 (KLF转录因子4)的相互作用,导致肌球蛋白轻链9 (MYL9)的表达增加。这种变化重塑了f -肌动蛋白,增强了肿瘤细胞的牵引力,从而促进了肿瘤的转移。靶向DDX3X-TLE2-MYL9通路可显著降低PDAC进展。本研究揭示了通过关注生物力学线索治疗PDAC的一种有前途的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


