Endoplasmic reticulum-mitochondria dual-targeting nanoplatform suppresses cancer stemness and enhances colorectal cancer immunotherapy
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Cancer stemness in colorectal cancer (CRC) drives immune evasion and fosters an immunosuppressive tumor microenvironment (TME), limiting the efficacy of immunotherapy. To counteract this stemness-driven immune evasion, we developed a hyaluronic acid (HA) surface-modified dual-targeting nanoplatform (HA/pCe6-Ato NPs) that induces both endoplasmic reticulum stress and mitochondrial dysfunction. HA functionalization facilitates tumor-specific targeting via CD44 receptor-mediated endocytosis. The nanoplatform incorporates an ER-targeting photosensitizer, pCe6, synthesized by conjugating Chlorin e6 with p-Toluenesulfonamide, and the mitochondrial respiratory chain inhibitor atovaquone (Ato), which self-assembles into nanoparticles. Upon activation, HA/pCe6-Ato NPs induce ER stress and mitochondrial dysfunction, disrupting cancer stemness and reprogramming the immunosuppressive TME. Preclinical evaluations showed that HA/pCe6-Ato NPs promote apoptosis and accelerate reactive oxygen species production in cancer stem-like cells (CSCs), while reducing immunosuppressive factor secretion and enhancing immune effector cell infiltration. These effects synergistically enhance the efficacy of immunotherapy, achieving superior tumor inhibition compared with conventional monotherapies. Collectively, HA/pCe6-Ato NPs highlight the potential of organelle-targeting strategies to dismantle cancer resilience mechanisms, providing a foundation for dual-targeting nanomedicines to overcome resistance and enhance immunotherapy in CRC.
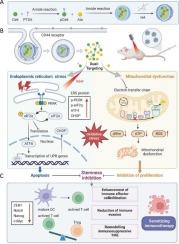
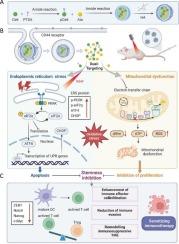
内质网-线粒体双靶向纳米平台抑制肿瘤干性并增强结直肠癌免疫治疗
结直肠癌(CRC)的癌症干细胞驱动免疫逃避并培养免疫抑制肿瘤微环境(TME),限制了免疫治疗的效果。为了对抗这种干细胞驱动的免疫逃避,我们开发了一种透明质酸(HA)表面修饰的双靶向纳米平台(HA/pCe6-Ato NPs),可诱导内质网应激和线粒体功能障碍。HA功能化通过CD44受体介导的内吞作用促进肿瘤特异性靶向。该纳米平台结合了一种er靶向光敏剂pCe6(由氯e6与对甲苯磺酰胺偶联合成)和线粒体呼吸链抑制剂阿托伐醌(Ato),后者可自组装成纳米颗粒。激活后,HA/pCe6-Ato NPs诱导内质网应激和线粒体功能障碍,破坏癌症干细胞并重新编程免疫抑制的TME。临床前评价表明,HA/pCe6-Ato NPs促进肿瘤干细胞(CSCs)凋亡,加速活性氧产生,同时减少免疫抑制因子分泌,增强免疫效应细胞浸润。这些效应协同增强了免疫治疗的疗效,与传统的单一治疗相比,实现了更好的肿瘤抑制。总的来说,HA/pCe6-Ato NPs强调了细胞器靶向策略在消除癌症恢复机制方面的潜力,为双靶向纳米药物克服CRC耐药和增强免疫治疗提供了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


