Design, synthesis, and biological evaluation of phenylsulfonylfuroxan-β-carboline-hydroxamic acid ternary hybrids for cancer
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
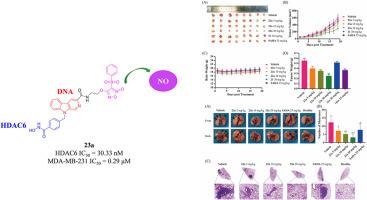
A total of 21 novel ternary hybrids containing phenylsulfonylfuroxan, β-carboline, and hydroxamic acid moieties were designed and synthesized. Their antitumor activities and underlying mechanisms of action were systematically evaluated. In vitro studies demonstrated that most of the target compounds exhibited moderate to potent antiproliferative activity. Among them, compound 23a showed the most promising activity against triple-negative breast cancer (TNBC) MDA-MB-231 cells, with a significant antiproliferative effect (IC50 = 0.29 μM) and favorable selectivity. Further investigations revealed that 23a significantly inhibited cell invasion and migration in MDA-MB-231 cells. Mechanistically, 23a exerted its antitumor effects through simultaneous targeting of HDAC6, DNA, and the release of high levels of nitric oxide (NO). Additionally, 23a induced apoptosis and caused G2/M phase cell cycle arrest. Pharmacokinetic parameters indicate that 23a possesses good absorption and rapid clearance in rat plasma, suggesting that it has suitable pharmacokinetic properties for antitumor activity in vivo. In vivo antitumor studies demonstrated that 23a achieved a superior tumor growth inhibition (54.49 %) compared to SAHA (34.16 %). In a Lewis lung carcinoma (LLC) metastatic mouse model, 23a significantly inhibited pulmonary metastasis formation. Moreover, 23a exhibited good safety profiles in ICR mice. These results collectively demonstrate that compound 23a represents a promising multi-target anticancer and anti-metastatic drug candidate with favorable development potential.
苯磺酰基呋喃-β-羰基-羟肟酸三元杂化物的设计、合成和生物学评价
设计并合成了21个新型三元杂化物,其中含有苯基磺酰基呋喃嘧啶、β-碳碱和羟肟酸基团。对其抗肿瘤活性及作用机制进行了系统评价。体外研究表明,大多数目标化合物表现出中等到强效的抗增殖活性。其中,化合物23a对三阴性乳腺癌(TNBC) MDA-MB-231细胞的抗增殖作用最显著(IC50 = 0.29 μM),且具有良好的选择性。进一步研究发现,23a显著抑制MDA-MB-231细胞的侵袭和迁移。机制上,23a通过同时靶向HDAC6、DNA和释放高水平一氧化氮(NO)来发挥其抗肿瘤作用。此外,23a诱导细胞凋亡,导致G2/M期细胞周期阻滞。药代动力学参数表明23a在大鼠血浆中具有良好的吸收和快速的清除率,表明23a在体内具有合适的抗肿瘤药代动力学特性。体内抗肿瘤研究表明,23a对肿瘤生长的抑制作用(54.49%)优于SAHA(34.16%)。在Lewis肺癌(LLC)转移小鼠模型中,23a显著抑制肺转移的形成。此外,23a在ICR小鼠中表现出良好的安全性。这些结果共同表明,化合物23a是一种具有良好开发潜力的多靶点抗癌和抗转移药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


