Unraveling the safety profile of GLP-1 receptor agonists: Mechanistic insights with a focus on semaglutide
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
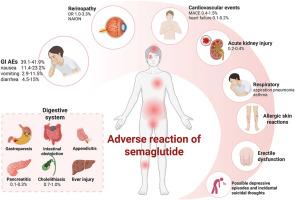
Semaglutide, a novel glucagon-like peptide-1 receptor agonist (GLP-1RA), has demonstrated remarkable therapeutic efficacy in managing type 2 diabetes (T2D) and obesity in recent years, emerging as a focal point in clinical research. However, with the expansion of its clinical application, there is increasing evidence that semaglutide can cause multisystem adverse reactions, leading to reduced patient compliance and safety. Recent research findings indicate that adverse events associated with semaglutide involve pathological changes across multiple systems (digestive, cardiovascular, neurological, reproductive, immune and respiratory) and organs (liver, kidneys, thyroid and retina). The precise mechanisms underlying these effects remain incompletely characterized, hindering early detection and targeted intervention in clinical practice. This review integrates evidence from preclinical studies, randomized controlled trials (RCTs), and real-world data to systematically evaluate the safety profile of GLP-1RAs, with semaglutide as the primary focus. It elucidates the biological mechanisms underlying organ/system-specific adverse effects broadly applicable to the GLP-1RA class. Furthermore, we propose a stratified management framework to guide personalized treatment strategies aligned with precision medicine principles, aiming to optimize the dynamic equilibrium between therapeutic efficacy and safety risks.

揭示GLP-1受体激动剂的安全性:以Semaglutide为重点的机制见解
Semaglutide是一种新型胰高血糖素样肽-1受体激动剂(GLP-1RA),近年来在治疗2型糖尿病(T2D)和肥胖方面表现出显著的疗效,成为临床研究的热点。然而,随着其临床应用的扩大,越来越多的证据表明,西马鲁肽可引起多系统不良反应,导致患者依从性和安全性降低。最近的研究结果表明,与semaglutide相关的不良事件涉及多个系统(消化、心血管、神经、生殖、免疫和呼吸)和器官(肝脏、肾脏、甲状腺和视网膜)的病理改变。这些影响的确切机制尚未完全确定,妨碍了临床实践中的早期发现和有针对性的干预。本综述整合了临床前研究、随机对照试验(rct)和真实世界数据的证据,系统地评估了GLP-1RAs的安全性,并以semaglutide为主要焦点。它阐明了广泛适用于GLP-1RA类的器官/系统特异性不良反应的生物学机制。此外,我们提出了一个分层管理框架,以指导符合精准医学原则的个性化治疗策略,旨在优化治疗效果和安全风险之间的动态平衡。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


