Advances in high-throughput drug screening based on pharmacotranscriptomics
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Background
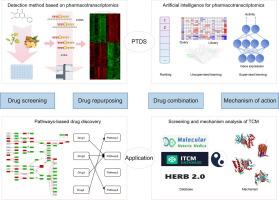
Drug screening constitutes the predominant paradigm for novel drug discovery. With the development of omics, drug screening has gradually developed into pharmacotranscriptomics-based drug screening (PTDS), which is different from target-based and phenotype-based drug screening. PTDS is a rapidly evolving interdisciplinary field that concurrently demands overcoming large-scale pharmacotranscriptomics profiling and computational challenges inherent to high-dimensional feature data.Aim of review: This review aims to summarize the developmental trajectory and research advancements in PTDS, with a focus on large-scale pharmacotranscriptomics profiling and artificial intelligence-driven data mining. It elucidates the appropriate application fields of PTDS in comparison to traditional drug screening paradigms, thereby providing novel perspectives for the technological evolution and implementation of PTDS.Key scientific concepts of review: PTDS can detect gene expression changes following drug perturbation in cells on a large scale and analyze the efficacy of drug-regulated gene sets, signaling pathways, and even complex diseases by combining artificial intelligence. The technical evolution of PTDS is systematically summarized, encompassing advancements in high-throughput PTDS detection technologies and data analysis methods. PTDS is categorized into microarray, targeted transcriptomics, and RNA-seq. Data analysis of PTDS involves ranking, unsupervised learning, and supervised learning algorithms. All these methods remain active in research and industry, coexisting to address evolving drug screening needs. On this basis, the roles of PTDS in promoting pathway-based drug screening strategies are deeply explored for drug discovery and drug combination design. Meanwhile, we also focus on the application of PTDS in screening and mechanism analysis of traditional Chinese medicine (TCM), which reflects that PTDS is suitable for detecting the complex efficacy of drugs, especially TCM. PTDS is an important development direction for high-throughput screening. By combining with artificial intelligence, PTDS will greatly revolutionize our understanding of drug screening and promote new drug research and development.
基于药物转录组学的高通量药物筛选研究进展
药物筛选是新药发现的主要范例。随着组学的发展,药物筛选逐渐发展为基于药物转录组学的药物筛选(PTDS),不同于基于靶标和基于表型的药物筛选。PTDS是一个快速发展的跨学科领域,同时需要克服大规模药物转录组学分析和高维特征数据固有的计算挑战。综述目的:本文综述了PTDS的发展历程和研究进展,重点介绍了PTDS的大规模药物转录组学分析和人工智能驱动的数据挖掘。通过与传统药物筛选模式的比较,阐述了PTDS的适用领域,为PTDS的技术发展和实施提供了新的视角。综述重点科学概念:PTDS可以大规模检测细胞内药物扰动后的基因表达变化,结合人工智能分析药物调控基因集、信号通路甚至复杂疾病的疗效。系统总结了PTDS的技术演变,包括高通量PTDS检测技术和数据分析方法的进展。PTDS分为微阵列、靶向转录组学和rna测序。PTDS的数据分析包括排序、无监督学习和监督学习算法。所有这些方法仍然活跃在研究和工业中,共存以满足不断变化的药物筛选需求。在此基础上,深入探讨PTDS在促进基于通路的药物筛选策略中的作用,用于药物发现和药物组合设计。同时,我们还重点介绍了PTDS在中药筛选和机制分析中的应用,这反映了PTDS适用于检测药物,特别是中药的复杂疗效。PTDS是高通量筛选的重要发展方向。通过与人工智能的结合,PTDS将极大地改变我们对药物筛选的认识,促进新药的研发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


