Efficient decomplexation of Cu(II) complexes and Cu(II) recovery via one-step Fenton-like oxidation/adsorption processes mediated by nanoconfined hydrous Co-Zr oxides
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
The removal of heavy metal complexes (HMCs) from wastewater is challenging due to their structural stability. Conventional advanced oxidation process (AOPs) requires the decomplexation and the subsequent adsorption to remove the released metal ions, which is relatively inefficient. This study proposes a one-step approach using Co-Zr-113/PMS for simultaneous oxidation-decomplexation and in-situ adsorption of Cu(II) complexes. This system exhibits varied oxidation performance and Cu(II) recovery efficiency towards different metal complexes (i.e., Cu-HEDP, Cu-Citrate, and Cu-EDTA) in the optimal pH between 6.0–8.0. Additionally, it was discovered that Co-Zr-113/PMS possesses excellent anti-interference capability with coexisting ions. Mechanistic studies indicated that HO• and SO4•− are proposed as the primary reactive species (PRS) involved in the degradation of Cu-Citrate and Cu-EDTA, whereas Co(II)-PMS complex acts as the PRS in the degradation of Cu-HEDP. The adsorption of Cu(II) complex is mainly achieved by forming Co/Zr-O-Cu and the carboxyl functional group (-COOH) of the D113 resin also plays a crucial role. Moreover, we verified that Co-Zr-113/PMS possesses excellent stability and repeatability through the adsorption and regeneration cycle experiments. Column adsorption experiments shows this system has practical application potential. This study presents a viable technology for the decomplexation of Cu(II) complexes and the recovery of Cu(II) from wastewater.
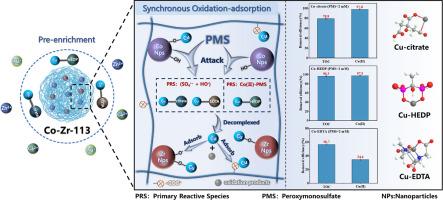
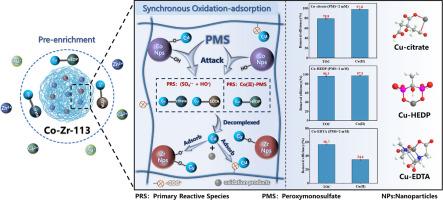
纳米限水Co-Zr氧化物介导的一步式fenton氧化/吸附过程中Cu(II)配合物的高效解合和Cu(II)的回收
重金属配合物(HMCs)由于其结构的稳定性,从废水中去除具有挑战性。传统的高级氧化工艺(AOPs)需要解复和随后的吸附来去除释放的金属离子,其效率相对较低。本研究提出了一种利用Co-Zr-113/PMS一步法同时进行Cu(II)配合物的氧化解合和原位吸附的方法。该体系对不同金属配合物(即Cu- hedp、Cu- citrate和Cu- edta)的氧化性能和Cu(II)回收效率在最佳pH为6.0 ~ 8.0之间。此外,还发现Co-Zr-113/PMS对共存离子具有良好的抗干扰能力。机理研究表明,HO•和SO4•−是参与Cu-Citrate和Cu-EDTA降解的主要反应物质(PRS),而Co(II)-PMS络合物则是Cu-HEDP降解的主要反应物质(PRS)。Cu(II)配合物的吸附主要通过形成Co/Zr-O-Cu来实现,D113树脂的羧基官能团(-COOH)也起着至关重要的作用。此外,我们通过吸附和再生循环实验验证了Co-Zr-113/PMS具有良好的稳定性和重复性。柱吸附实验表明,该体系具有实际应用潜力。本研究提出了一种可行的铜(II)络合物解解和废水中铜(II)回收技术。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


