Intravenous lipid-siRNA conjugate mediates gene silencing at the blood-brain barrier and blood-CSF barrier
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
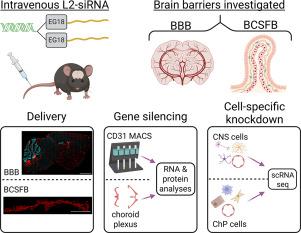
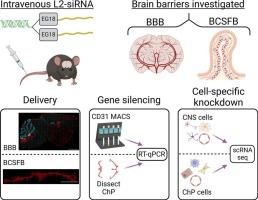
Barriers of the central nervous system (CNS), such as the blood-brain barrier (BBB) and blood-cerebrospinal fluid barrier (BCSFB), regulate the two-way exchange of material between the blood and CNS. While the BBB and BCSFB can become dysfunctional in patients with chronic CNS diseases, few studies have focused on strategies for targeting these interfaces. Here, we showed that an intravenously administered lipid-siRNA conjugate was delivered to and silenced genes within brain endothelial cells and choroid plexus epithelial cells, which comprise the BBB and BCSFB, respectively. A single intravenous dose of lipid-siRNA conjugate was delivered to ∼100 % of brain endothelial cells and major choroid plexus cell types, without substantial delivery into brain parenchymal tissue. Sustained mRNA and protein silencing was achieved in both brain endothelial cells and bulk choroid plexus tissues. Moreover, single cell RNA sequencing demonstrated gene knockdown in capillaries, venous endothelial cells, and choroid plexus epithelial cells without silencing genes in parenchymal cell populations. Collectively, this work establishes an effective nonviral framework to mediate gene inhibition in the brain barriers.
静脉注射脂质- sirna偶联物介导血脑屏障和血脑脊液屏障的基因沉默
中枢神经系统(CNS)的屏障,如血脑屏障(BBB)和血脑脊液屏障(BCSFB),调节血液和中枢神经系统之间的双向物质交换。虽然血脑屏障和BCSFB在慢性中枢神经系统疾病患者中可能功能失调,但很少有研究关注针对这些界面的策略。在这里,我们展示了静脉给药脂质- sirna偶联物被递送到脑内皮细胞和脉络膜丛上皮细胞内并沉默基因,它们分别由血脑屏障和BCSFB组成。单次静脉注射剂量的脂质- sirna偶联物被递送到~100 %的脑内皮细胞和主要脉络膜丛细胞类型,没有大量递送到脑实质组织。在脑内皮细胞和大块脉络膜丛组织中均实现了mRNA和蛋白的持续沉默。此外,单细胞RNA测序显示,在实质细胞群体中,毛细血管、静脉内皮细胞和脉络膜丛上皮细胞中基因敲低而没有基因沉默。总的来说,这项工作建立了一个有效的非病毒框架来介导脑屏障中的基因抑制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


