Vascular endothelial cell-targeted mRNA delivery via synthetic lipid nanoparticles for venous thrombosis prevention
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Venous thromboembolism significantly contributes to the global disease burden. Anticoagulant and antiplatelet therapies are currently the treatment strategies. However, challenges remain due to hemorrhagic complications and the inability to resolve established thrombi. There is an urgent need for a new generation of antithrombotic agents. Given the fibrin specificity and rapid thrombus dissolution capacity of recombinant tissue plasminogen activator (TPA) protein, along with the significant advantages of mRNA therapeutics in protein replacement, we aim to develop an antithrombotic strategy through the targeted delivery of TPA mRNA to vascular endothelial cells using synthetic lipid nanoparticles (LNPs). A series of amino ionizable lipids were synthesized to create an LNP library, from which the LNP selectively targeting vascular endothelial cells (vtLNP) was selected by a DNA barcode labeling high-throughput screening method. The antithrombotic efficacy and safety of vtLNP loaded with TPA mRNA (vtLNP@TPA) were evaluated in a deep vein thrombosis (DVT) mouse model and normal ICR mice, respectively. The results revealed that vtLNP@TPA significantly prevented the occurrence and development of venous thrombosis. This study provides relevant experimental evidence for a novel antithrombotic therapy strategy for venous thrombosis using mRNA therapeutics.
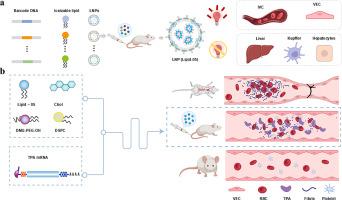
通过合成脂质纳米颗粒靶向血管内皮细胞mRNA递送预防静脉血栓形成
静脉血栓栓塞显著增加了全球疾病负担。抗凝和抗血小板治疗是目前的治疗策略。然而,由于出血性并发症和无法解决已建立的血栓,挑战仍然存在。迫切需要新一代的抗血栓药物。考虑到重组组织纤溶酶原激活物(TPA)蛋白的纤维蛋白特异性和快速溶栓能力,以及mRNA治疗在蛋白质替代中的显著优势,我们的目标是通过使用合成脂质纳米颗粒(LNPs)靶向递送TPA mRNA到血管内皮细胞来开发一种抗血栓策略。合成一系列氨基离化脂质,构建LNP文库,通过DNA条形码标记高通量筛选方法,从中筛选出选择性靶向血管内皮细胞(vtLNP)的LNP。分别在深静脉血栓形成(DVT)小鼠模型和正常ICR小鼠中评估负载TPA mRNA的vtLNP的抗血栓疗效和安全性(vtLNP@TPA)。结果显示vtLNP@TPA对静脉血栓形成的发生和发展有明显的预防作用。本研究为利用mRNA治疗静脉血栓的新型抗血栓治疗策略提供了相关实验证据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


