Antisense oligonucleotide-loaded nanozyme reverses tumor immune suppression through sonogenetic metabolic therapy
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The immunosuppressive adenosine generated during immunogenic cell death (ICD) attenuates the ICD-elicited antitumor immune responses, while hypoxia-induced overexpression of CD73 in solid tumors exacerbates adenosine accumulation. Herein, a pioneering sonogenetic metabolic therapy was developed to activate ICD while inhibiting adenosine production. Specifically, a metal-organic framework (MOF) incorporating Ru single-atom catalytic sites was engineered to achieve high-affinity sonosensitizer loading, which was further functionalized with mPEG-d-PEI for efficient delivery of antisense oligonucleotides (ASOs) targeting CD73 mRNA. The designed system exhibited three-tiered therapeutic amplification: Ru-based catalytic sites facilitated atom-economic conversion of tumor-overproduced H₂O₂ into oxygen, alleviating tumor hypoxia. Sustained oxygen supply amplified sonodynamic effect by generating robust ROS to induce tumor apoptosis and ICD, while concurrently suppressing HIF-1α-driven CD73 upregulation. Ultrasound-responsive lysosomal disruption combined with PEI-mediated interference enabled effective lysosomal escape of ASOs, downregulating CD73 expression to inhibit adenosine production. Through immune-metabolic reprogramming of the tumor microenvironment, the approach significantly inhibited tumor growth while establishing long-term immune memory to combat pulmonary metastases in mice. Notably, beyond serving as an antitumor strategy, the developed oligonucleotide delivery system remodels metabolic homeostasis by targeting key components in signaling pathways, thereby providing new perspectives for oligonucleotide-based therapies in metabolic disease treatment.
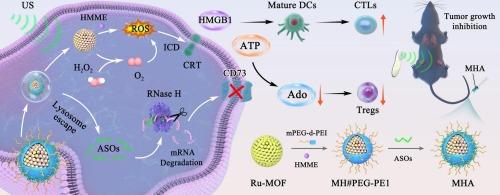
反义寡核苷酸负载纳米酶通过声源代谢疗法逆转肿瘤免疫抑制
免疫原性细胞死亡(ICD)过程中产生的免疫抑制腺苷会减弱ICD引发的抗肿瘤免疫反应,而实体瘤中缺氧诱导的CD73过表达会加剧腺苷的积累。在此,开发了一种开创性的超声代谢疗法来激活ICD,同时抑制腺苷的产生。具体来说,设计了一个包含Ru单原子催化位点的金属有机框架(MOF)来实现高亲和力的声敏剂负载,并通过mPEG-d-PEI进一步功能化,以有效递送靶向CD73 mRNA的反义寡核苷酸(ASOs)。设计的系统具有三级治疗放大作用:ru基催化位点促进肿瘤过量产生的h2o2转化为氧气的原子经济转化,缓解肿瘤缺氧。持续供氧可通过产生强大的ROS来放大声动力效应,诱导肿瘤凋亡和ICD,同时抑制hif -1α驱动的CD73上调。超声反应性溶酶体破坏结合pei介导的干扰使ASOs有效的溶酶体逃逸,下调CD73的表达以抑制腺苷的产生。通过肿瘤微环境的免疫代谢重编程,该方法显著抑制肿瘤生长,同时建立长期免疫记忆来对抗小鼠肺转移。值得注意的是,除了作为一种抗肿瘤策略外,所开发的寡核苷酸递送系统通过靶向信号通路中的关键成分来重塑代谢稳态,从而为基于寡核苷酸的代谢性疾病治疗提供了新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


