Design, synthesis and in vitro assessment of di-/tri-methoxy-aryl-substituted mono-spiro-1,2,4,5-tetraoxanes for antiplasmodial and anticancer use
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
In the present article, a library of novel non-symmetrical di-/tri-methoxy-aryl-substituted mono-spiro-1,2,4,5-tetraoxanes (5a-m) has been prepared from methyltrioxorhenium(VII) complex-catalysed two-step one-pot direct oxidation approach. The in vitro antiplasmodial and cytotoxic potentials of all non-symmetrical di-/tri-methoxy-aryl-substituted mono-spiro-1,2,4,5-tetraoxanes (5a-m) have been assessed against the chloroquine-resistant FcB1 strain of Plasmodium falciparum, HeLa, A549 and A2780 cancer cells. The in vitro biological assessment has afforded eight di-/tri-methoxy-aryl-substituted mono-spiro-1,2,4,5-tetraoxane derivatives (5b-e, 5g, 5i, 5k-l) with nanomolar antiplasmodial activity (IC50 = 34–91 nM and Selectivity Index = 109–2900). Along with this, two tri-OMe-aryl substituted mon-spiro-1,2,4,5-tetraoxane analogues (5i and 5l) have also shown dual potency with strong antiplasmodial (5i, IC50 = 46 nM; 5l, IC50 = 48 nM), and in vitro micromolar cytotoxicity against the A2780 ovarian cancer (5i, IC50 = 3.82 μM; 5l, IC50 = 1.98 μM) and HeLa cells (5i, LD50 = 10.85 μM; 5l, LD50 = 5.8 μM). In addition, potential drug-target interactions prediction for representative compounds (5d, 5i, and 5l) has been examined using different computational-based techniques. Through this study, the selective incorporation of functionalized di-/tri-methoxy-aryl moieties on the mon-spiro-1,2,4,5-tetraoxane skeleton has proved efficient in improving the overall biological activities of the resulting molecules.
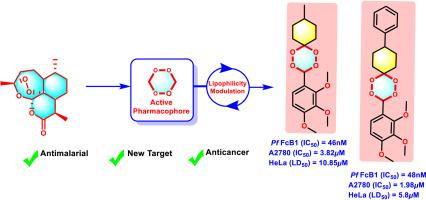
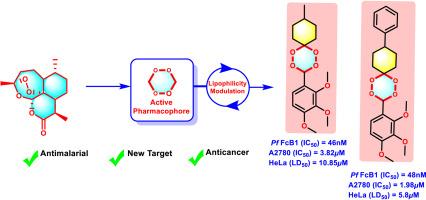
抗疟原虫和抗癌用二/三甲氧基芳基取代单螺-1,2,4,5-四氧烷的设计、合成及体外评价
本文采用甲基三氧鎓(VII)配合物催化两步一锅直接氧化法制备了新型非对称二/三甲氧基芳基取代单螺-1,2,4,5-四氧烷(5 -m)。研究了所有非对称二/三甲氧基芳基取代单螺-1,2,4,5-四氧烷(5a-m)对恶性疟原虫、HeLa、A549和A2780耐氯喹的FcB1株癌细胞的体外抗疟原虫和细胞毒性。体外生物学评价得到8个二/三甲氧基芳基取代单螺-1,2,4,5-四氧烷衍生物(5b-e, 5g, 5i, 5k-l)具有纳米摩尔抗疟原虫活性(IC50 = 34-91 nM,选择性指数= 109-2900)。与此同时,两种三烯丙基取代的单螺-1,2,4,5-四氧烷类似物(5i和5l)也显示出双效,具有较强的抗疟原虫作用(5i, IC50 = 46nM; 5l, IC50 = 48nM),以及对A2780卵巢癌(5i, IC50 = 3.82μM; 5l, IC50 = 1.98μM)和HeLa细胞(5i, LD50 = 10.85μM; 5l, LD50 = 5.8μM)的体外微摩尔细胞毒性。此外,使用不同的基于计算的技术对代表性化合物(5d, 5i和5l)的潜在药物-靶标相互作用进行了预测。通过本研究,选择性地将功能化的二-/三甲氧基芳基部分结合到非螺-1,2,4,5-四氧烷骨架上,可以有效地提高所得分子的整体生物活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


