Modulating glucose homeostasis via expression of PPAR-γ TF: Pharmacological insights into quinolone-based hydrazones
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
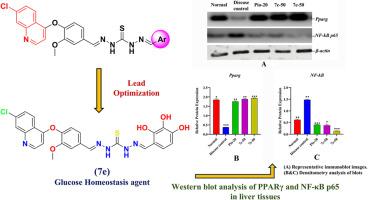
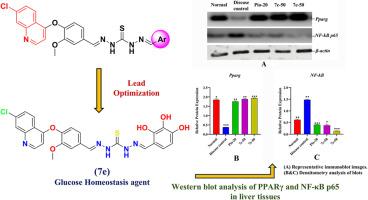
The growing prevalence of type 2 diabetes underscores the urgent need for novel therapeutic agents targeting glucose homeostasis and insulin sensitivity. In this study, a new series of quinolone-based hydrazone derivatives (7a–7k) was synthesized and evaluated for their ability to modulate peroxisome proliferator-activated receptor gamma (PPAR-γ), a key regulator of glucose and lipid metabolism. Among them, compounds 7c and 7e showed strong, concentration-dependent activation of PPAR-γ in HepG2 and L6 myotube cell lines, comparable to pioglitazone. In vivo studies using STZ-nicotinamide-induced diabetic rats confirmed their efficacy, with 7e significantly lowering fasting blood glucose, improving glucose tolerance, and restoring metabolic balance. Histopathological analysis revealed protection of pancreatic islets, hepatocytes, skeletal muscle, and adipose tissue. Molecular studies further demonstrated upregulation of Pparg, Glut4, and AdipoQ, alongside suppression of TNF-α, IL-6, and NF-κB p65, highlighting both insulin-sensitizing and anti-inflammatory effects. Docking and 100 ns molecular dynamics simulations validated the stable binding of 7c and 7e within the PPAR-γ ligand-binding domain. Collectively, these findings identify 7c and 7e as promising multifunctional candidates for type 2 diabetes management through dual regulation of glucose homeostasis and inflammation.
通过表达PPAR-γ TF调节葡萄糖稳态:喹诺酮类腙的药理学见解
随着2型糖尿病患病率的增加,迫切需要针对葡萄糖稳态和胰岛素敏感性的新型治疗药物。在这项研究中,合成了一系列新的喹诺酮类腙衍生物(7a-7k),并评估了它们调节过氧化物酶体增殖激活受体γ (PPAR-γ)的能力,PPAR-γ是葡萄糖和脂质代谢的关键调节因子。其中,化合物7c和7e在HepG2和L6肌管细胞系中表现出强烈的、浓度依赖性的PPAR-γ激活作用,与吡格列酮相当。stz -烟酰胺诱导的糖尿病大鼠体内实验证实了其疗效,7e显著降低空腹血糖,改善糖耐量,恢复代谢平衡。组织病理学分析显示胰岛、肝细胞、骨骼肌和脂肪组织具有保护作用。分子研究进一步证实了Pparg、Glut4和AdipoQ的上调,以及TNF-α、IL-6和NF-κB p65的抑制,突出了胰岛素增敏和抗炎作用。对接和100 ns分子动力学模拟验证了7c和7e在PPAR-γ配体结合域中的稳定结合。总的来说,这些发现确定7c和7e是通过葡萄糖稳态和炎症的双重调节来治疗2型糖尿病的有希望的多功能候选者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


