Bilayer self-assembly encapsulated by engineered vesicle disrupts glutathione to induce Disulfidptosis-enhanced cuproptosis for tumor immunotherapy
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Cuproptosis, a copper-dependent programmed cell death, can effectively activate tumor immunogenicity and reverse the immunosuppressive tumor microenvironment (TME). Nevertheless, the overexpression of xCT-SLC7A11 in tumor cells is beneficial for maintaining the biosynthesis of glutathione (GSH) to counteract cuproptosis. Herein, copper-based bilayer self-assembly Cel-Cu/DHA (CCD) encapsulated by red blood cell vesicle engineered with folate (RF), CCD@RF nanoparticles (NPs), have been prepared to target synergistic therapeutic mechanisms and induce disulfidptosis-enhanced cuproptosis for augmented anti-tumor immunotherapy by disrupting intracellular GSH balance. In xCT-SLC7A11high cancer cells, dihydroartemisinin (DHA) pre-released from CCD@RF NPs serves to impede glucose metabolism, thereby downregulating NADPH levels, causing the accumulation of cystine and the collapse of actin cytoskeleton proteins. This simultaneously induces disulfidptosis and blocks the source of cysteine which is essential for GSH synthesis, thereby amplifying cuproptosis with assistance of celastrol (Cel). Moreover, Cu2+ released from CCD@RF NPs, once reduced by GSH, could catalyze Fenton-like reactions to generate hydroxyl radicals (·OH), further disrupting intracellular redox homeostasis. Tumor cells undergoing immunogenic cell death (ICD) derived from disulfidptosis-enhanced cuproptosis, could release antigens, inducing robust immune responses, ultimately effectively inhibiting tumor growth and metastasis. This work proposes a novel dual-pronged therapeutic strategy by disrupting intracellular redox balance, offering a new direction for future anti-tumor immunotherapy.
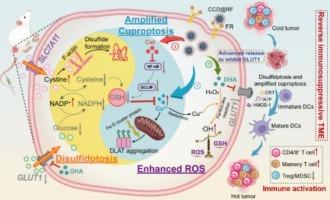

由工程囊泡封装的双分子层自组装破坏谷胱甘肽,诱导双硫化物增强的铜突起用于肿瘤免疫治疗
铜增生是一种铜依赖性程序性细胞死亡,可有效激活肿瘤免疫原性,逆转免疫抑制性肿瘤微环境(TME)。然而,xCT-SLC7A11在肿瘤细胞中的过度表达有利于维持谷胱甘肽(GSH)的生物合成以对抗铜变形。本文制备了一种铜基双分子层自组装细胞- cu /DHA (CCD),由叶酸(RF)修饰的红细胞囊泡包裹,CCD@RF纳米颗粒(NPs),旨在靶向协同治疗机制,并通过破坏细胞内GSH平衡诱导二硫酸化增强铜沉降,从而增强抗肿瘤免疫治疗。在xct - slc7a11高表达的癌细胞中,CCD@RF NPs预释放的双氢青蒿素(DHA)可抑制葡萄糖代谢,从而下调NADPH水平,导致胱氨酸的积累和肌动蛋白骨架蛋白的崩溃。这同时诱导双曲下垂并阻断对GSH合成至关重要的半胱氨酸的来源,从而在celastrol (Cel)的帮助下放大铜下垂。此外,CCD@RF NPs释放的Cu2+一旦被GSH还原,可以催化芬顿样反应产生羟基自由基(·OH),进一步破坏细胞内氧化还原稳态。肿瘤细胞因双硫化物作用增强的cuprotosis而发生免疫原性细胞死亡(immunogenic cell death, ICD),可释放抗原,诱导强大的免疫应答,最终有效抑制肿瘤生长和转移。这项工作提出了一种新的双管齐下的治疗策略,通过破坏细胞内氧化还原平衡,为未来的抗肿瘤免疫治疗提供了新的方向。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


