Neutrophil extracellular traps: Formation, pathological roles, and nanoparticle-based therapeutic targeting strategies
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Neutrophil extracellular traps (NETs) are large, web-like DNA structures released by neutrophils, coated with histones and antimicrobial proteins. They serve as a crucial defense mechanism for neutrophils against microbial invasion, playing a significant role in eliminating microorganisms such as bacteria, fungi, and viruses. While NETs are primarily recognized for their role in microbial defense, growing evidence indicates that excessive NET formation, triggered by physical and chemical stimuli, pathogens, or pathological factors, can worsen inflammation and cause organ damage. Understanding NETs' presence in various tissues and body fluids is crucial for elucidating their contribution to disease etiopathogenesis. By designing nanoparticles that can either prevent NET formation or facilitate their degradation, researchers aim to mitigate the harmful effects of excessive NETs. These nanotechnological interventions can be tailored to specifically target the molecular components of NETs, enhancing treatment precision and efficacy. Furthermore, nanoparticles can deliver therapeutic agents directly to inflammation sites, reducing systemic side effects and improving patient outcomes. This review summarizes the role of NETs in various pathologies, focusing on strategies to inhibit NETosis, including mechanisms of pathogen evasion, and the use of nanodelivery systems to enhance the efficiency of NETs inhibition or removal.
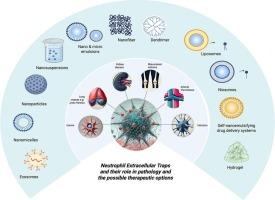
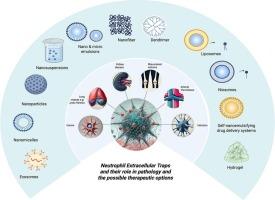
中性粒细胞胞外陷阱:形成,病理作用,和纳米粒子为基础的治疗靶向策略
中性粒细胞胞外陷阱(NETs)是由中性粒细胞释放的大型网状DNA结构,被组蛋白和抗菌蛋白包裹。它们是中性粒细胞抵御微生物入侵的重要防御机制,在消灭细菌、真菌和病毒等微生物方面发挥着重要作用。虽然NET主要被认为在微生物防御中起作用,但越来越多的证据表明,由物理和化学刺激、病原体或病理因素引发的NET的过量形成可加重炎症并导致器官损伤。了解NETs在各种组织和体液中的存在对于阐明它们在疾病发病机制中的作用至关重要。通过设计可以阻止NET形成或促进其降解的纳米颗粒,研究人员旨在减轻过量NET的有害影响。这些纳米技术干预措施可以专门针对NETs的分子成分,从而提高治疗精度和疗效。此外,纳米颗粒可以将治疗剂直接输送到炎症部位,减少全身副作用,改善患者预后。本文综述了NETs在各种病理中的作用,重点介绍了抑制NETosis的策略,包括病原体逃避的机制,以及利用纳米递送系统提高NETs抑制或去除的效率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


