Antimicrobial core-shell nanofiber wound dressing with chloramphenicol-liposomes
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
In the context of a rising incidence of chronic wounds worldwide, developing more efficient wound dressings is in demand. In this setting, nanofiber dressings have shown promising features. We combined three biopolymers: beta-glucan (βG), chitosan (CHI) and pectin into the same nanofibers. In addition, antimicrobial activity was provided adding chloramphenicol (CAM), which also was entrapped in liposomes for a more sustained drug release. Applying a coaxial electrospinning setup allowed fabricating core-shell nanofibers, with βG and CHI in the shell, and pectin with CAM liposomes in the core. Initially, challenges during electrospinning with gel-clogging of the needle tip emerged, due to the formation of a polyelectrolyte complex between the positively charged CHI and negatively charged pectin. This happened although pectin and CHI were separated in the coaxial electrospinning setup. Here, the critical intervention to solve this problem was reducing the pH of the pectin-containing core-solution. The successful electrospun core-shell nanofibers (Coax-LipCAM), with a mean diameter of around 200 nm, was confirmed by SEM and TEM images. Among the control nanofibers, a mono-axial control-nanofiber, Mono-core-CAM, was prepared. This control formulation contained the same polymers as present in the core of the Coax-LipCAM together with “free” CAM (not entrapped in liposomes). When Mono-core-CAM was compared with Coax-LipCAM, Coax-LipCAM exhibited enhanced tensile strength and higher stability in simulated wound fluid. Furthermore, CAM release from Coax-LipCAM was extended, with 80 % of the drug released after eight hours. Finally, all CAM-containing nanofibers showed antimicrobial activity comparable to pure CAM when tested against Escherichia coli and S. aureus. In conclusion, core-shell nanofibers with CAM-liposomes in a pectin-core and with a shell contained βG and CHI, were successfully prepared. Their promising morphological and mechanical characteristics, favorable stability and swelling properties, sustained CAM release, and preserved antimicrobial activity encourages further clinical evaluation targeting the treatment of chronic wounds.
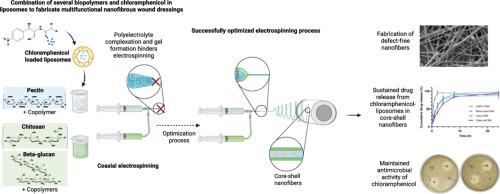
氯霉素脂质体抗菌核壳纳米纤维创面敷料。
在世界范围内慢性伤口发病率不断上升的背景下,需要开发更有效的伤口敷料。在这种情况下,纳米纤维敷料显示出有希望的特性。我们将三种生物聚合物:β -葡聚糖(βG),壳聚糖(CHI)和果胶结合到相同的纳米纤维中。此外,添加氯霉素(CAM)提供了抗菌活性,氯霉素也被包裹在脂质体中,以获得更持久的药物释放。采用同轴静电纺丝装置可以制造出核-壳纳米纤维,壳中有βG和CHI,果胶中有CAM脂质体。最初,由于在带正电的CHI和带负电的果胶之间形成了多电解质复合物,静电纺丝过程中出现了针尖凝胶堵塞的问题。尽管果胶和CHI在同轴静电纺丝装置中分离,但仍发生了这种情况。在这里,解决这个问题的关键干预措施是降低含有果胶的核心溶液的pH值。通过扫描电镜(SEM)和透射电镜(TEM)对制备的Coax-LipCAM纳米纤维进行了验证,其平均直径约为200 nm。在控制纳米纤维中,制备了单轴控制纳米纤维Mono-core-CAM。该对照制剂含有与Coax-LipCAM核心相同的聚合物以及“自由”CAM(未被脂质体包裹)。当与Coax-LipCAM进行比较时,Coax-LipCAM在模拟伤口液中表现出更高的抗拉强度和更高的稳定性。此外,Coax-LipCAM的CAM释放被延长,8小时后80%的药物释放。最后,所有含CAM的纳米纤维对大肠杆菌和金黄色葡萄球菌的抗菌活性与纯CAM相当。综上所述,我们成功地制备了含有cam -脂质体的果胶核和含有βG和CHI的核-壳纳米纤维。它们具有良好的形态学和力学特性,良好的稳定性和消肿特性,持续的CAM释放,以及保留的抗菌活性,鼓励进一步的临床评估,针对慢性伤口的治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


