Direct Z-scheme Hf2CO2/MoSSe van der Waals heterostructure for photocatalytic water splitting: high solar-to-hydrogen efficiency and excellent carrier mobility
IF 9.5
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Photocatalytic water splitting for hydrogen production is crucial for the sustainable development of global energy resources. In this work, we employ first-principles calculations to construct a direct Z-scheme Hf2CO2/MoSSe heterostructure and systematically investigate its photocatalytic performance. The results indicate that the Hf2CO2/MoSSe heterostructure facilitates photocatalytic water splitting within a pH range of 0 to 10, demonstrating excellent pH tolerance, and exhibits a peak optical absorption of 2 × 105 cm−1 in the visible light range. The maximum solar-to-hydrogen (STH) efficiency reaches 22.52%, increasing to 34.48% with application of a +2% biaxial tensile strain. Applying an −8% biaxial compressive strain enhances the peak optical absorption within the visible light range by 30% compared to the intrinsic heterostructure. Furthermore, the carrier mobility of the heterostructure exhibits distinct anisotropy, with the electron and hole mobilities reaching 2767 cm2 V−1 s−1 and 4287 cm2 V−1 s−1, respectively, facilitating efficient spatial separation and rapid migration of photogenerated carriers. The calculation and screening to determine optimal adsorption sites for intermediate products reveal a Gibbs free energy change (ΔG) of 1.37 eV for the Hydrogen Evolution Reaction (HER), while the Oxygen Evolution Reaction (OER) proceeds spontaneously under illumination. These results all indicate that the Hf2CO2/MoSSe heterostructure is a highly promising candidate material for photocatalytically driven overall water splitting for hydrogen production.
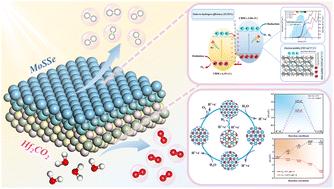
直接Z-scheme Hf2CO2/MoSSe范德华异质结构用于光催化水分解:高太阳能制氢效率和优异的载流子迁移率
光催化水裂解制氢技术对全球能源的可持续发展至关重要。在这项工作中,我们采用第一性原理计算构建了直接Z-scheme Hf2CO2/MoSSe异质结构,并系统地研究了其光催化性能。结果表明,Hf2CO2/MoSSe异质结构在0 ~ 10的pH范围内有利于光催化水分解,具有良好的pH耐受性,在可见光范围内光吸收峰值为2 × 105 cm−1。最大太阳能制氢效率达到22.52%,当施加+2%的双轴拉伸应变时,太阳能制氢效率提高到34.48%。与本征异质结构相比,施加−8%的双轴压缩应变可使可见光范围内的峰值光吸收提高30%。此外,异质结构的载流子迁移率表现出明显的各向异性,电子和空穴迁移率分别达到2767 cm2 V−1 s−1和4287 cm2 V−1 s−1,有利于光生载流子的有效空间分离和快速迁移。计算和筛选中间产物的最佳吸附位点表明,析氢反应(HER)的吉布斯自由能变化(ΔG)为1.37 eV,而析氧反应(OER)在光照下自发进行。这些结果都表明,Hf2CO2/MoSSe异质结构是一种非常有前途的光催化全水裂解制氢材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Materials Chemistry A
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
19.50
自引率
5.00%
发文量
1892
审稿时长
1.5 months
期刊介绍:
The Journal of Materials Chemistry A, B & C covers a wide range of high-quality studies in the field of materials chemistry, with each section focusing on specific applications of the materials studied. Journal of Materials Chemistry A emphasizes applications in energy and sustainability, including topics such as artificial photosynthesis, batteries, and fuel cells. Journal of Materials Chemistry B focuses on applications in biology and medicine, while Journal of Materials Chemistry C covers applications in optical, magnetic, and electronic devices. Example topic areas within the scope of Journal of Materials Chemistry A include catalysis, green/sustainable materials, sensors, and water treatment, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


