Charge separation engineering via CoAl2O4/ZnCdS heterojunction and d-band center modulation for synergistically enhanced photocatalytic hydrogen evolution
IF 9.5
2区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In response to the bottleneck of traditional photocatalysts, this study innovatively constructed a nanoflower-structured CoAl2O4-loaded ZnCdS solid solution (CZ15) composite photocatalyst, achieving an improvement in the performance of solar-driven hydrogen production. The hydrogen production rate of CZ15 under visible light is as high as 7388.72 μmol g−1 h−1, which is nearly 5.24 times higher than that of single ZnCdS, and its activity does not decline after 4 cycles. The improvement of performance stems from the synergistic effect of multiple mechanisms: a type-II heterojunction formed at the interface between CoAl2O4 and ZnCdS effectively drives the migration of photogenerated electrons from ZnCdS to CoAl2O4, thereby significantly suppressing electron–hole recombination; meanwhile, the introduction of CoAl2O4 regulates the electronic structure of the composite catalyst. DFT calculations confirmed that the center of its d-band was closer to the Fermi level, which optimizes the adsorption energy of the reaction intermediates and accelerates the surface reaction kinetics. In addition, the unique CoAl2O4 nanoflower structure effectively increases the specific surface area and provides more active sites.
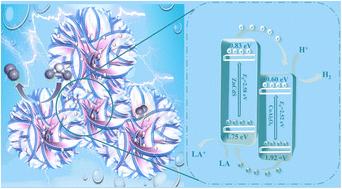
基于CoAl2O4/ZnCdS异质结和d波段中心调制的电荷分离工程协同增强光催化析氢
针对传统光催化剂的瓶颈,本研究创新构建了一种纳米花状结构的负载coal2o4的ZnCdS固溶体(CZ15)复合光催化剂,实现了太阳能制氢性能的提升。CZ15在可见光下的产氢速率高达7388.72 μmol g−1 h−1,是单一ZnCdS的近5.24倍,且经过4次循环后活性不下降。性能的提高源于多种机制的协同作用:在CoAl2O4与ZnCdS的界面处形成ii型异质结,有效地驱动了光生电子从ZnCdS向CoAl2O4的迁移,从而显著抑制了电子-空穴复合;同时,CoAl2O4的加入调节了复合催化剂的电子结构。DFT计算证实其d带中心更接近费米能级,优化了反应中间体的吸附能,加速了表面反应动力学。此外,独特的CoAl2O4纳米花结构有效地增加了比表面积,提供了更多的活性位点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Materials Chemistry A
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
19.50
自引率
5.00%
发文量
1892
审稿时长
1.5 months
期刊介绍:
The Journal of Materials Chemistry A, B & C covers a wide range of high-quality studies in the field of materials chemistry, with each section focusing on specific applications of the materials studied. Journal of Materials Chemistry A emphasizes applications in energy and sustainability, including topics such as artificial photosynthesis, batteries, and fuel cells. Journal of Materials Chemistry B focuses on applications in biology and medicine, while Journal of Materials Chemistry C covers applications in optical, magnetic, and electronic devices. Example topic areas within the scope of Journal of Materials Chemistry A include catalysis, green/sustainable materials, sensors, and water treatment, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


