Klotho attenuates D-galactose-induced cardiac aging through the ROS/NLRP3/pyroptosis pathway
IF 4.7
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Objective
Activation of NLRP3 inflammasome contributes to cardiac aging progression. Klotho, a recognised anti-aging protein, exerts protective effects against cardiac aging. In this study, we aimed to elucidate the protective effects of Klotho on D-galactose (D-gal)-induced cardiac aging and the underlying mechanisms.
Methods
Aging severity in mice was evaluated based on coat condition and serum Klotho levels. Serum levels of interleukin (IL)-1β, lactate dehydrogenase (LDH), superoxide dismutase (SOD), and malondialdehyde were measured to assess cardiac oxidative stress and inflammatory response damage. Cardiac function was evaluated using echocardiography, whereas heart histopathological changes were observed through haematoxylin-eosin (HE) staining, Masson staining, and heart index. Cardiac aging was further assessed with β-galactosidase staining and western blot analysis of aging-related proteins (P53 and P21). Pyroptosis-related protein expression was assessed via western blot, and cardiac tissue reactive oxygen species (ROS) expression levels were determined through dihydroethidium staining. Similar analyses were conducted on D-gal-treated H9C2 cardiomyocytes.
Results
Compared to wild-type aged mice, Klotho-treated and NLRP3 knockout mice showed markedly reduced back hair loss, elevated serum Klotho and SOD levels, reduced serum IL-1β and LDH, enhanced left ventricular ejection fraction, left ventricular fractional shortening, peak E to peak A ratio, diminished heart size, cardiomyocyte hypertrophy and collagen deposition. Decreased cardiac aging markers, apoptosis-associated speck-like protein (ASC) formation, NLRP3 expression, cleaved-caspase-1, gasdermin D (GSDMD), IL-1β, and IL-18, and lower ROS levels were observed in cardiac tissues. These protective effects were abolished upon Nigericin injection.
Conclusions
Klotho delays D-gal-induced cardiac aging by regulating the ROS/NLRP3/pyroptosis pathway.
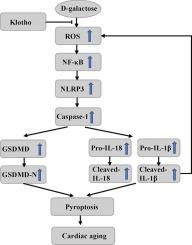
Klotho通过ROS/NLRP3/焦亡途径减缓d -半乳糖诱导的心脏衰老。
目的:NLRP3炎性体的激活与心脏衰老进程有关。Klotho是一种公认的抗衰老蛋白,对心脏衰老具有保护作用。在本研究中,我们旨在阐明Klotho对d -半乳糖(D-gal)诱导的心脏衰老的保护作用及其机制。方法:以被毛状况和血清Klotho水平评价小鼠衰老严重程度。测定血清白细胞介素(IL)-1β、乳酸脱氢酶(LDH)、超氧化物歧化酶(SOD)和丙二醛水平,以评估心脏氧化应激和炎症反应损伤。采用超声心动图评价心脏功能,通过血红素-伊红(HE)染色、Masson染色和心脏指数观察心脏组织病理变化。通过β-半乳糖苷酶染色和衰老相关蛋白(P53和P21)的western blot分析进一步评估心脏衰老。western blot检测焦热相关蛋白表达,双氢乙啶染色检测心脏组织活性氧(ROS)表达水平。对d -gal处理的H9C2心肌细胞进行了类似的分析。结果:与野生型老年小鼠相比,Klotho处理和NLRP3基因敲除小鼠的背部脱发明显减少,血清Klotho和SOD水平升高,血清IL-1β和LDH降低,左心室射血分数增加,左心室分数缩短,E峰与A峰比值增加,心脏大小减小,心肌细胞肥大,胶原沉积明显减少。心脏老化标志物、凋亡相关斑点样蛋白(ASC)形成、NLRP3表达、裂解caspase-1、气皮蛋白D (GSDMD)、IL-1β和IL-18降低,心脏组织中ROS水平降低。注射尼日利亚菌素后,这些保护作用消失。结论:Klotho通过调节ROS/NLRP3/焦亡通路延缓d -gal诱导的心脏衰老。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
0.00%
发文量
171
审稿时长
42 days
期刊介绍:
The Journal of Molecular and Cellular Cardiology publishes work advancing knowledge of the mechanisms responsible for both normal and diseased cardiovascular function. To this end papers are published in all relevant areas. These include (but are not limited to): structural biology; genetics; proteomics; morphology; stem cells; molecular biology; metabolism; biophysics; bioengineering; computational modeling and systems analysis; electrophysiology; pharmacology and physiology. Papers are encouraged with both basic and translational approaches. The journal is directed not only to basic scientists but also to clinical cardiologists who wish to follow the rapidly advancing frontiers of basic knowledge of the heart and circulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


