Results of TOR001: An open-label single patient study using targeted bacteriophage therapy for the treatment of a chronic urinary tract infection
IF 4.6
2区 医学
Q1 INFECTIOUS DISEASES
International Journal of Antimicrobial Agents
Pub Date : 2025-09-08
DOI:10.1016/j.ijantimicag.2025.107613
引用次数: 0
Abstract
Chronic urinary tract infections are persistent bacterial infections with the potential to drive antibiotic resistance. Like other persistent bacterial infections, intracellular bacterial reservoirs and biofilm formation hinder the clearance of pathogens despite long courses of antibiotic therapy. New strategies for treatment of these persistent infections are needed.
Here we describe the results of an open-label individual patient study using bacteriophage therapy to treat a chronic urinary tract infection in an immunocompetent 72-year-old woman without underlying urolithiasis or indwelling devices. We co-administered HP3.1, HP3, and ES19 bacteriophages with demonstrated in vitro activity via bladder instillation, orally, and as a topical formulation.
The primary outcome was safety and tolerability of the treatment. Adverse events were monitored through daily symptom logs and laboratory analysis of blood samples obtained throughout the study. We found that the treatment was safe and well tolerated with no serious adverse events reported. No adverse events were deemed related to the study material. The secondary outcomes were clinical and microbiological efficacy as monitored through daily symptom logs and standard urine culture, respectively. Two weeks after initial clinical improvement, her condition relapsed and culture was consistent with previous isolates. Treatment with ertapenem following bacteriophage therapy led to sustained clinical and microbiologic cure. Exploratory analysis through whole genome sequencing of pre- and post-treatment isolates identified mutations in genes associated with adhesion and evasion that may influence virulence and promote clearance.
These results inform expanded randomized clinical trials and support the growing literature that bacteriophage therapies are safe and effective.
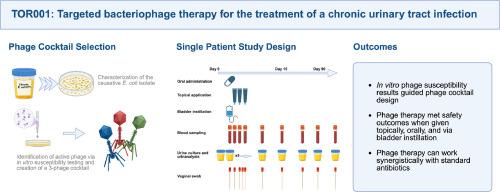
TOR001的结果:一项使用靶向噬菌体治疗慢性尿路感染的开放标签单患者研究。
慢性尿路感染是一种持续的细菌感染,有可能导致抗生素耐药性。像其他持续性细菌感染一样,细胞内细菌储存库和生物膜的形成阻碍了病原体的清除,尽管长期的抗生素治疗。需要新的策略来治疗这些持续性感染。在这里,我们描述了一项开放标签个体患者研究的结果,该研究使用噬菌体疗法治疗一名免疫功能正常的72岁妇女的慢性尿路感染,该妇女没有潜在的尿石症或留置装置。我们通过膀胱滴注、口服和外用制剂的方式共同给药HP3.1、HP3和ES19噬菌体,这些噬菌体具有体外活性。主要结果是治疗的安全性和耐受性。通过每日症状记录和整个研究过程中获得的血液样本的实验室分析来监测不良事件。我们发现治疗是安全且耐受性良好的,没有严重的不良事件报告。没有不良事件被认为与研究材料有关。次要结果分别是通过每日症状记录和标准尿液培养监测的临床和微生物效果。在最初的临床改善两周后,她的病情复发,培养与以前的分离株一致。在噬菌体治疗后用厄他培南治疗导致持续的临床和微生物治疗。通过对治疗前和治疗后分离株的全基因组测序进行探索性分析,发现了与粘附和逃避相关的基因突变,这些突变可能影响毒力并促进清除。这些结果为扩大随机临床试验提供了信息,并支持了噬菌体治疗安全有效的文献。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
21.60
自引率
0.90%
发文量
176
审稿时长
36 days
期刊介绍:
The International Journal of Antimicrobial Agents is a peer-reviewed publication offering comprehensive and current reference information on the physical, pharmacological, in vitro, and clinical properties of individual antimicrobial agents, covering antiviral, antiparasitic, antibacterial, and antifungal agents. The journal not only communicates new trends and developments through authoritative review articles but also addresses the critical issue of antimicrobial resistance, both in hospital and community settings. Published content includes solicited reviews by leading experts and high-quality original research papers in the specified fields.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


