Estradiol alleviates disease phenotypes caused by m.3635G > a mutations by activating mitochondrial biogenesis and PINK1-Parkin mediated mitophagy in iPSC-derived retinal pigment epithelium cells.
IF 3.7
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
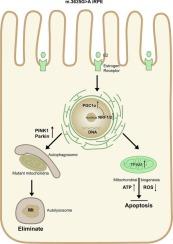
Leber's hereditary optic neuropathy (LHON), a mitochondrial disorder marked by central vision loss, exhibits incomplete penetrance and male predominance. Since there are no adequate models for understanding the rapid vision loss associated with LHON, we generated induced pluripotent stem cells (iPSCs) from LHON patients carrying the pathogenic m.3635G > A mutation and differentiated them into retinal pigment epithelium (RPE) cells. The mutation disrupted mitochondrial dynamics, suppressing OPA1-mediated fusion and enhancing DRP1-dependent fission, resulting in decreased expression of ND1, ND5, NDUFB8, SDHB and COX2, impaired mitochondrial bioenergetic function, and cell proliferation. Additionally, the m.3635G > A mutation promoted intrinsic apoptosis, altered autophagic flux, evidenced by elevating levels in apoptotic proteins PARP1, caspase-3, and 9, reduced levels of autophagy protein LC3-II, and increased levels of substrate P62. Moreover, the m.3635G > A mutation inhibited PINK1-Parkin-dependent mitophagy. Based on sex-specific differences in hormone metabolism, we proposed that estrogen plays a protective role in women and showed that estrogen receptor α and β were downregulated in LHON. We demonstrated that estradiol improved cell viability by reducing apoptosis, inducing mitochondrial biogenesis through the PGC1α-NRF1/2-TFAM axis, and vigorously promoting PINK1-Parkin-dependent mitophagy in LHON iPSCs and iPSC-derived RPE cells. Our findings have highlighted the critical role of the m.3635G > A mutation in the pathogenetic process of LHON, and our observations support the hypothesis that estrogen is helpful in the preventive treatment of LHON.
雌二醇通过激活线粒体生物发生和PINK1-Parkin介导的ipsc源性视网膜色素上皮细胞的线粒体自噬,减轻由m.3635G > a突变引起的疾病表型。
Leber's遗传性视神经病变(LHON)是一种以中央视力丧失为特征的线粒体疾病,表现出不完全外显性和男性优势。由于没有足够的模型来理解与LHON相关的快速视力丧失,我们从携带致病性m.3635G > A突变的LHON患者中获得诱导多能干细胞(iPSCs),并将其分化为视网膜色素上皮(RPE)细胞。该突变破坏了线粒体动力学,抑制了opa1介导的融合,增强了drp1依赖的裂变,导致ND1、ND5、NDUFB8、SDHB和COX2的表达降低,线粒体生物能量功能受损,细胞增殖受损。此外,m.3635G > A突变促进内源性凋亡,改变自噬通量,凋亡蛋白PARP1、caspase-3和9水平升高,自噬蛋白LC3-II水平降低,底物P62水平升高。此外,m.3635G > A突变抑制了pink1 - parkin依赖性的有丝分裂。基于激素代谢的性别差异,我们提出雌激素在女性中发挥保护作用,并表明雌激素受体α和β在LHON中下调。我们证明雌二醇通过减少凋亡,通过PGC1α-NRF1/2-TFAM轴诱导线粒体生物发生,并在LHON iPSCs和ipsc衍生的RPE细胞中大力促进pink1 - parkin依赖性的线粒体自噬,从而提高细胞活力。我们的研究结果强调了m.3635G > A突变在LHON发病过程中的关键作用,我们的观察结果支持了雌激素有助于LHON预防性治疗的假设。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


