From BN-Dewar benzene to BN-benzvalene: a computational exploration of photoisomerization mechanisms
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
This study explores the photochemical conversion of BN-Dewar benzene into BN-benzvalene derivatives, offering a strategic route to heteroatom-containing valence isomers with distinctive electronic properties. Using time-dependent density functional theory (TD-DFT) and electron localization function (ELF) analyses, the excited-state mechanism and associated structural rearrangements were elucidated. Vertical excitation to the S1 state was found to weaken the CC and B–N bonds while strengthening the N–Si bond in silyl-substituted derivatives, a key factor enabling efficient BN-benzvalene formation. Two minimum energy conical intersections MECI1 and MECI2 govern the deactivation pathways: MECI1 promotes irreversible C2–B bond cleavage and C1–B bond formation, driving the system toward BN-Benzvalene, whereas MECI2 enables relaxation back to the BN-Dewar benzene reactant. Nitrogen substitution, particularly with trialkylsilyl groups, significantly enhances the reaction yield by stabilizing charge redistribution and lowering Franck–Condon excitation energies. Nonradiative decay via MECI1 proceeds barrierlessly, favoring the production of BN-benzvalene. Finally, ELF analysis reveals that bond formation occurs through electron density migration rather than via radical intermediates.
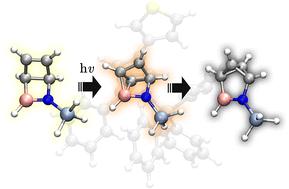
从bn -杜瓦苯到bn -苯苯:光异构化机制的计算探索。
本研究探索了bn -杜瓦苯光化学转化为bn -苯二苯衍生物,为获得具有独特电子性质的含杂原子价异构体提供了一条战略途径。利用时变密度泛函理论(TD-DFT)和电子定位函数(ELF)分析,阐明了激发态机制和相关的结构重排。研究发现,在硅基取代衍生物中,垂直激发到S1态会削弱C - c_ - C和B-N键,而增强N-Si键,这是有效生成bn -苯二烯的关键因素。MECI1和MECI2两个最小能量的圆锥形交叉点控制着失活途径:MECI1促进不可逆的C2-B键断裂和C1-B键形成,驱动体系向bn -苯戊烯方向发展,而MECI2使体系松弛回bn -杜瓦苯反应物。氮取代,特别是三烷基硅基取代,通过稳定电荷再分配和降低frank - condon激发能,显著提高了反应产率。通过MECI1进行的非辐射衰变无障碍进行,有利于bn -苯甲醛的产生。最后,ELF分析表明,键的形成是通过电子密度迁移而不是通过自由基中间体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


