Phenazines contribute to microbiome dynamics by targeting topoisomerase IV
IF 19.4
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Phenazines are highly prevalent, natural bioactive substances secreted by microbes. However, their mode of action and potential involvement in shaping microbiomes remain elusive. Here we performed a comprehensive analysis of over 1.35 million bacterial genomes to identify phenazine-producing bacteria distributed across 193 species in 34 families. Analysis of rhizosphere microbiome and public rhizosphere metagenomic datasets revealed that phenazines could shape the microbial community by inhibiting Gram-positive bacteria, which was verified by pairwise interaction assays using Phenazine-1-carboxamide (PCN)-producing Pseudomonas chlororaphis. PCN induced DNA damage in Bacillus subtilis, a model Gram-positive target, where it directly bound to the bacterial topoisomerase IV, inhibiting its decatenation activity and leading to cell death. A two-species consortium of phenazine-producing Pseudomonas and resistant B. subtilis exhibited superior synergistic activity in preventing Fusarium crown rot in wheat plants. This work advances our understanding of a prevalent microbial interaction and its potential for biocontrol. Computational analysis and pairwise assays reveal that bacterial topoisomerase IV is a target of microbially produced phenazines, which informs synthetic-community design to treat fungal crop infection.

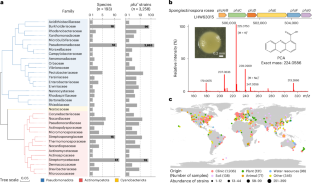
非那嗪类药物通过靶向拓扑异构酶IV促进微生物组动力学
非那嗪是由微生物分泌的一种非常普遍的天然生物活性物质。然而,它们的作用模式和在塑造微生物组中的潜在参与仍然难以捉摸。在这里,我们对超过135万个细菌基因组进行了全面分析,以确定分布在34个科193个物种中的产非那嗪细菌。对根际微生物组和公共根际宏基因组数据集的分析显示,非那嗪可以通过抑制革兰氏阳性菌来塑造微生物群落,这一点通过使用产生PCN的绿假单胞菌进行两两相互作用实验得到了证实。PCN诱导模型革兰氏阳性靶点枯草芽孢杆菌DNA损伤,直接与细菌拓扑异构酶IV结合,抑制其十癸二烯化活性,导致细胞死亡。产非那嗪假单胞菌和耐药枯草芽孢杆菌在防治小麦镰刀菌冠腐病中表现出较强的协同作用。这项工作促进了我们对普遍存在的微生物相互作用及其生物防治潜力的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


