Decoding the gene expression response of Cerastoderma edule to chronic trematode infection: A comparison among host tissues
IF 2.4
3区 生物学
Q1 ZOOLOGY
引用次数: 0
Abstract
Parasites can induce gene expression changes in their hosts, either benefiting the parasite or the host. In particular, trematodes are not only one of the most ubiquitous groups of aquatic parasites, they also have huge impacts on individual hosts with significant ecological and economic repercussions. The trematode Bucephalus minimus infects Cerastoderma edule (the edible cockle), a socioeconomically and ecologically important bivalve, as its first intermediate host. This parasite is one of the most harmful parasites infecting cockles, affecting their ability to reproduce, grow, and survive, thereby indirectly impacting ecosystem functioning. Despite the well-documented ecological effects of B. minimus, its impacts at a molecular level remain poorly understood. This study aimed to investigate the molecular mechanisms underlying B. minimus infection in cockles by analysing tissue-specific and systemic responses to long-term parasitic infection. It compared gene expression profiles in two critical tissues of naturally infected and non-infected cockles: the digestive gland, the primary target of infection, and the haemolymph, the backbone of the bivalve immune system. Results revealed extensive tissue-specific changes in gene expression. In the haemolymph, infected cockles showed significant downregulation of pathways related to cell division, cytoskeletal organization, and DNA repair, suggesting potentially parasite-induced reduction of immune responses and host cellular functions. Contrary to expectations, immune pathways did not show significantly increased expression, likely reflecting the chronic nature of infection and energy reallocation by the host. In the digestive gland, genes associated with gametogenesis, metabolism and immune function were downregulated, with no significant upregulation observed, except in some genes related to scavenger receptor activity and inflammation, suggesting localized immune responses. Shared responses among tissues included alterations in zinc ion transport and neurotransmitter biosynthesis, suggesting management of infection-induced stress. These findings highlight how B. minimus may manipulate host biology to suppress immunity and disrupt critical cellular processes, providing valuable insights into chronic trematode infections and host-parasite dynamics.
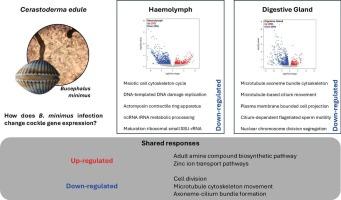
解码荚膜荚膜对慢性吸虫感染的基因表达反应:宿主组织间的比较。
寄生虫可以诱导宿主的基因表达变化,这对寄生虫和宿主都有好处。特别是吸虫不仅是最普遍存在的水生寄生虫之一,而且对个体宿主具有巨大的影响,具有显著的生态和经济影响。吸虫小Bucephalus感染了一种社会经济和生态上重要的双壳类动物——贝壳贝(Cerastoderma edule)作为它的第一个中间宿主。这种寄生虫是感染蛤的最有害的寄生虫之一,影响其繁殖、生长和生存的能力,从而间接影响生态系统的功能。尽管小芽孢杆菌的生态效应有充分的文献记载,但其在分子水平上的影响仍然知之甚少。本研究旨在通过分析贝对长期寄生虫感染的组织特异性和全身反应,探讨贝感染微小贝氏杆菌的分子机制。研究比较了自然感染和未感染蛤贝的两个关键组织的基因表达谱:消化腺(感染的主要目标)和血淋巴(双壳类免疫系统的骨干)。结果显示基因表达发生了广泛的组织特异性变化。在血淋巴中,感染的鸟蛤显示出与细胞分裂、细胞骨架组织和DNA修复相关的通路的显著下调,这表明寄生虫可能诱导免疫反应和宿主细胞功能的降低。与预期相反,免疫通路并没有显示出显著的表达增加,这可能反映了宿主感染和能量重新分配的慢性性质。在消化腺中,与配子发生、代谢和免疫功能相关的基因下调,除一些与清道夫受体活性和炎症相关的基因外,未观察到明显的上调,提示局部免疫反应。组织间的共同反应包括锌离子转运和神经递质生物合成的改变,提示感染诱导应激的管理。这些发现强调了小b虫如何操纵宿主生物学来抑制免疫和破坏关键的细胞过程,为慢性吸虫感染和宿主-寄生虫动力学提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.10
自引率
5.90%
发文量
94
审稿时长
1 months
期刊介绍:
The Journal of Invertebrate Pathology presents original research articles and notes on the induction and pathogenesis of diseases of invertebrates, including the suppression of diseases in beneficial species, and the use of diseases in controlling undesirable species. In addition, the journal publishes the results of physiological, morphological, genetic, immunological and ecological studies as related to the etiologic agents of diseases of invertebrates.
The Journal of Invertebrate Pathology is the adopted journal of the Society for Invertebrate Pathology, and is available to SIP members at a special reduced price.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


