Catalytic asymmetric reactions of isocyanides for constructing non-central chirality.
IF 2.1
4区 化学
Q2 CHEMISTRY, ORGANIC
Beilstein Journal of Organic Chemistry
Pub Date : 2025-08-19
eCollection Date: 2025-01-01
DOI:10.3762/bjoc.21.129
引用次数: 0
Abstract
Beyond the conventional carbon-centered chirality, catalytic asymmetric transformations of isocyanides have recently emerged as a powerful strategy for the efficient synthesis of structurally diverse scaffolds featuring axial, planar, helical, and inherent chirality. Herein, we summarize the exciting achievements in this rapidly evolving field. These elegant examples have been organized and presented based on the reaction type as well as the resulting chirality form. Additionally, we provide a perspective on the current limitations and future opportunities, aiming to inspire further advances in this area.
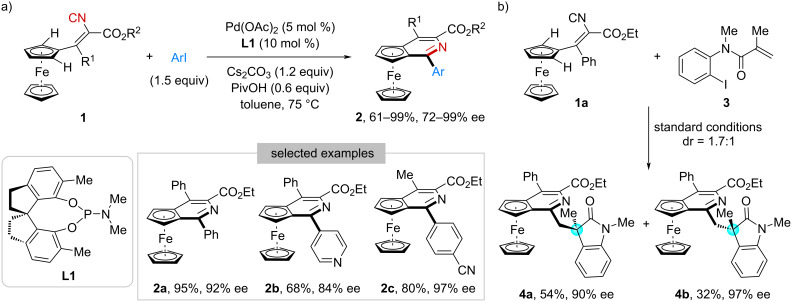
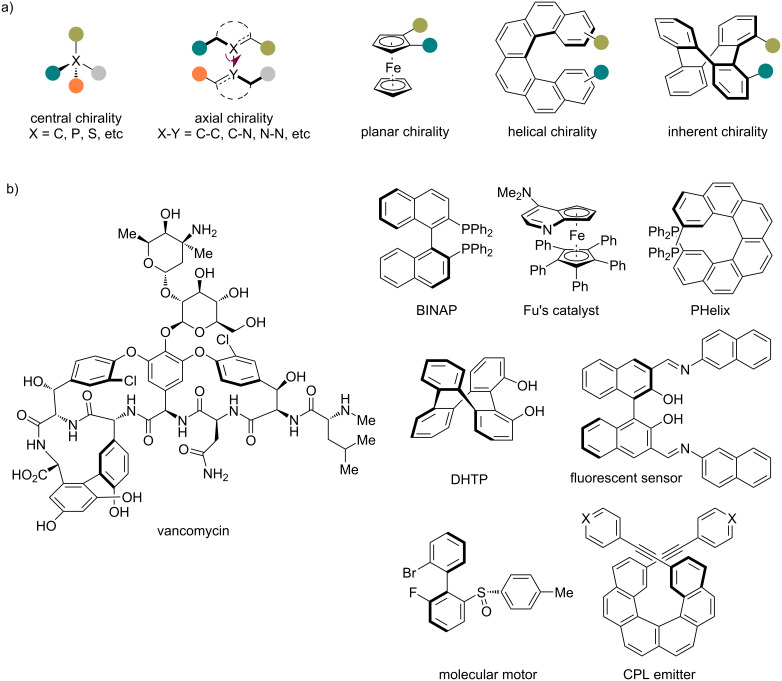
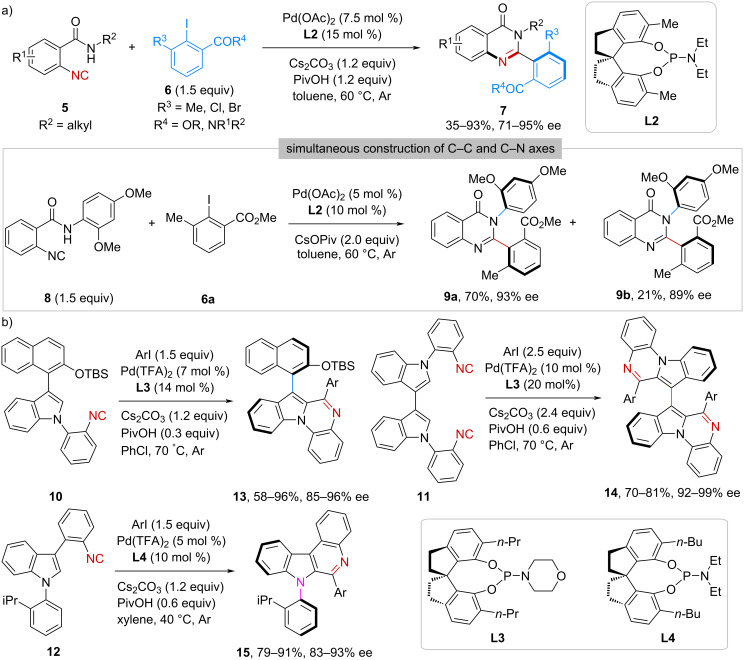
异氰酸酯非中心手性的催化不对称反应。
除了传统的碳中心手性外,催化异氰酸酯的不对称转化最近成为一种有效合成具有轴向、平面、螺旋和固有手性的结构多样化支架的有力策略。在此,我们总结了在这个快速发展的领域中令人兴奋的成就。这些优雅的例子是根据反应类型和产生的手性形式来组织和呈现的。此外,我们还提供了当前局限性和未来机会的观点,旨在激发该领域的进一步发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
4.90
自引率
3.70%
发文量
167
审稿时长
1.4 months
期刊介绍:
The Beilstein Journal of Organic Chemistry is an international, peer-reviewed, Open Access journal. It provides a unique platform for rapid publication without any charges (free for author and reader) – Platinum Open Access. The content is freely accessible 365 days a year to any user worldwide. Articles are available online immediately upon publication and are publicly archived in all major repositories. In addition, it provides a platform for publishing thematic issues (theme-based collections of articles) on topical issues in organic chemistry.
The journal publishes high quality research and reviews in all areas of organic chemistry, including organic synthesis, organic reactions, natural product chemistry, structural investigations, supramolecular chemistry and chemical biology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


