4H-3,1-Benzoxazin-4-ones Instead of Isatoic Anhydride: Reducing the Activity of the Substrate Allows You to Reduce the Synthesis Time
IF 0.8
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A method was developed for one-step preparing 5-(2-acylaminophenyl)-3-aryloxadiazoles by the reaction of amidoximes with benzoxazines, which does not require elevated temperatures and a long reaction time. Using a strong basic medium—a solution of NaOH in DMSO—allows for rapid condensation of the intermediately formed O-acylamidoximes at room temperature. Compared with a similar method for the synthesis of unsubstituted 5-(2-aminophenyl)oxadiazoles based on the isatoic anhydride reaction, the proposed one-step procedure not only makes it possible to obtain acyl derivatives of these amino heterocycles, but also reduces the reaction time 20-fold.
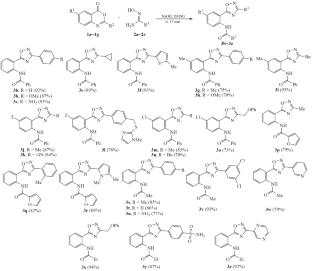
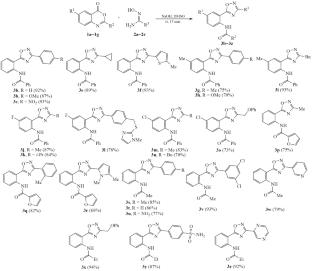
用4h -3,1-苯并恶嗪-4-酮代替异核酸酐:降低底物活性可以缩短合成时间
建立了偕胺肟与苯并恶嗪一步反应制备5-(2-酰基氨基苯基)-3-芳基二唑的方法,该方法不需要高温和长时间的反应。使用强碱介质——氢氧化钠在dmso中的溶液——可以使中间形成的o -酰基胺肟在室温下快速缩合。与基于异核酸酐反应的非取代5-(2-氨基苯基)恶二唑的类似合成方法相比,该一步法不仅可以得到这些氨基杂环的酰基衍生物,而且将反应时间缩短了20倍。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.40
自引率
22.20%
发文量
252
审稿时长
2-4 weeks
期刊介绍:
Russian Journal of General Chemistry is a journal that covers many problems that are of general interest to the whole community of chemists. The journal is the successor to Russia’s first chemical journal, Zhurnal Russkogo Khimicheskogo Obshchestva (Journal of the Russian Chemical Society ) founded in 1869 to cover all aspects of chemistry. Now the journal is focused on the interdisciplinary areas of chemistry (organometallics, organometalloids, organoinorganic complexes, mechanochemistry, nanochemistry, etc.), new achievements and long-term results in the field. The journal publishes reviews, current scientific papers, letters to the editor, and discussion papers.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


