Cross-linked chitosan-argan nutshell bio-composite beads: Optimization using Box-Behnken design and adsorption mechanism for Pb (II) and Cd (II) removal
Q3 Materials Science
引用次数: 0
Abstract
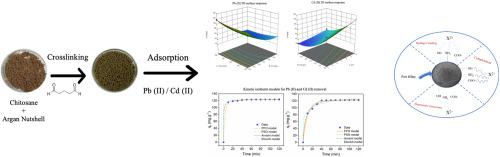
Industrial heavy metal contamination in water poses a significant threat to both the environment and human health, necessitating the development of affordable and effective remediation solutions. This study introduces a novel chitosan–argan nutshell bio-composite bead cross-linked in situ with glutaraldehyde (CS/ANS@GA), which exhibits enhanced mechanical stability and serves as an eco-friendly adsorbent for the efficient removal of Pb (II) and Cd(II). The bio-composite beads were thoroughly characterized using swelling tests, XRD, FTIR, and SEM-EDX, confirming their semi-crystalline structure and functionalities, as well as their high porosity and accessible adsorption active sites. Response surface methodology was employed to optimize the effects of pH, adsorbent dose, and contact time to achieve high removal efficiencies of Pb (II) and Cd (II). The synthesized beads exhibited Sips isotherm behavior, indicating a heterogeneous surface with maximum adsorption capacities of 433 mg g−1 (Pb) and 391 mg.g−1 (Cd). Thermodynamic analysis revealed an endothermic and spontaneous process, while Avrami kinetics suggested a complex adsorption mechanism involving pore diffusion, electrostatic interactions, and hydrogen bonding. Remarkably, the CS/ANS@GA beads maintained an efficiency of over 90 % after three adsorption–desorption cycles. These results highlight the potential of CS/ANS@GA beads as a sustainable, high-performance material for removing heavy metals from water.
交联壳聚糖-坚果壳生物复合微球:Box-Behnken设计优化吸附Pb (II)和Cd (II)的机理
水中的工业重金属污染对环境和人类健康构成重大威胁,因此有必要制定负担得起的有效补救办法。本研究介绍了一种新型的壳聚糖-坚果果壳生物复合材料-戊二醛原位交联(CS/ANS@GA),该材料具有增强的机械稳定性,是一种高效去除Pb (II)和Cd(II)的环保吸附剂。通过膨胀测试、XRD、FTIR和SEM-EDX对生物复合微珠进行了全面表征,证实了它们的半晶结构和功能,以及它们的高孔隙率和可接近的吸附活性位点。采用响应面法优化了pH、吸附剂剂量和接触时间对Pb (II)和Cd (II)去除效果的影响。合成的微球表现出Sips等温行为,表明其表面不均匀,最大吸附量为433 mg g−1 (Pb)和391 mg g−1 (Cd)。热力学分析表明吸附过程为吸热自发过程,而Avrami动力学分析表明吸附机制复杂,包括孔隙扩散、静电相互作用和氢键。值得注意的是,经过三次吸附-解吸循环后,CS/ANS@GA微球的效率保持在90%以上。这些结果突出了CS/ANS@GA微珠作为一种可持续的高性能材料去除水中重金属的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JCIS open
Physical and Theoretical Chemistry, Colloid and Surface Chemistry, Surfaces, Coatings and Films
CiteScore
4.10
自引率
0.00%
发文量
0
审稿时长
36 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


