Influence of wettability on diffusion limited nanoparticle adsorption at gas-liquid interfaces
Q3 Materials Science
引用次数: 0
Abstract
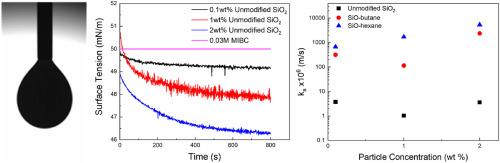
This study investigates the influence of hydrophobicity on particle adsorption by examining the behavior of hydrophobized silica particles at air-water interfaces. Langmuir-Blodgett (LB) trough studies of butanol (‘SiO-butane’) and hexanol (‘SiO-hexane’) esterified particles provided contrasting behavior. The SiO-butane particles formed weaker particle layers that underwent partial collapse with compression, leading to formations significantly below hexagonal close-packed estimates. In contrast, the SiO-hexane particles exhibited improved monolayer behavior and longer-range stability. Droplet surface tensions demonstrated that the hydrophobic particles significantly altered the dynamic tension during adsorption, when methyl isobutyl carbinol (MIBC) was added as a co-surfactant. Short-term modeling elucidated the role of diffusion and energy barriers on adsorption dynamics, with SiO-hexane having reduced diffusion coefficients with respect to SiO-butane and unmodified particles. Despite this reduced diffusion, long-term modeling allowed calculation of adsorption coefficients (ka), which for SiO-hexane particles were ∼200 × greater than for unmodified particles at low 0.1 wt% particle concentrations and over 1000 × greater at 2 wt%. Overall, the results provide quantitative insights into the profound influence of hydrophobicity on particle adsorption, particularly in crowded surface environments. Importantly, a diffusion-only mechanism is inadequate to explain adsorption dynamics for these larger colloids and the gravity-driven contribution must be considered in early-stage kinetics.
润湿性对扩散受限纳米颗粒在气液界面吸附的影响
本研究通过观察疏水性二氧化硅颗粒在空气-水界面的行为,探讨了疏水性对颗粒吸附的影响。Langmuir-Blodgett (LB)通过研究丁醇(‘ sio -丁烷’)和己醇(‘ sio -己烷’)酯化颗粒提供了对比的行为。sio -丁烷颗粒形成了较弱的颗粒层,这些颗粒层在压缩过程中经历了部分坍塌,导致地层明显低于六方紧密堆积的估计。相比之下,sio -己烷颗粒表现出更好的单层行为和更长的范围稳定性。液滴表面张力表明,当加入甲基异丁基甲醇(MIBC)作为助表面活性剂时,疏水颗粒在吸附过程中显著改变了动态张力。短期模型阐明了扩散和能量势垒对吸附动力学的作用,硅己烷相对于硅丁烷和未修饰的颗粒具有降低的扩散系数。尽管这种扩散减少了,但长期建模允许计算吸附系数(ka),在低0.1 wt%的颗粒浓度下,sio -己烷颗粒的吸附系数比未改性颗粒大200倍,在2 wt%的颗粒浓度下,吸附系数比未改性颗粒大1000倍以上。总的来说,这些结果为疏水性对颗粒吸附的深刻影响提供了定量的见解,特别是在拥挤的表面环境中。重要的是,仅扩散机制不足以解释这些较大胶体的吸附动力学,在早期动力学中必须考虑重力驱动的贡献。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JCIS open
Physical and Theoretical Chemistry, Colloid and Surface Chemistry, Surfaces, Coatings and Films
CiteScore
4.10
自引率
0.00%
发文量
0
审稿时长
36 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


