Osteoimmunomodulation of astragalus-calcium silicate scaffolds-activated M2 macrophage-derived miR-218-rich exosome for enhanced bone regeneration
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Current therapeutic strategies for bone defects, including autografts, allografts, and conventional biomaterial scaffolds, are limited by donor site morbidity, immune rejection, and insufficient vascularization. Moreover, the complex inflammatory microenvironment in bone defects often impairs healing outcomes, necessitating the development of advanced biomaterials with enhanced immunomodulatory and regenerative capabilities. This study investigates the therapeutic potential of extracellular vesicles derived from Astragalus-modified calcium silicate (AstCS)-stimulated M2 macrophages (AstCSM2EVs) in bone regeneration. The AstCSM2EVs demonstrated superior immunomodulatory capabilities by effectively polarizing macrophages toward the M2 phenotype, characterized by significant downregulation of pro-inflammatory cytokines (IL-1β, TNF-α) and concurrent upregulation of anti-inflammatory mediators (IL-4, IL-10). Notably, AstCSM2EVs exhibited enhanced angiogenic potential, evidenced by increased endothelial tube formation and elevated VEGF secretion, while simultaneously promoting osteogenic differentiation of mesenchymal stem cells through upregulated expression of key markers including ALP, BSP, and OC. Mechanistic investigations revealed that AstCSM2EVs modulated these regenerative processes primarily through miR-218-5p-mediated regulation of multiple signaling pathways, including NOD-like receptor and ECM-receptor interaction pathways. In a rabbit femoral defect model, local administration of AstCSM2EVs significantly enhanced bone regeneration, demonstrated by increased bone volume fraction and improved trabecular architecture, while effectively suppressing local inflammation. These findings establish AstCSM2EVs as a promising therapeutic agent for bone regeneration, highlighting their multifaceted roles in immunomodulation, angiogenesis, and osteogenesis. This research introduces an innovative approach that combines extracellular vesicles (EV) with immunomodulatory tissue engineering strategies to improve the treatment of bone defects.
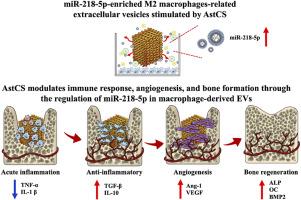
黄芪-硅酸钙支架活化的M2巨噬细胞来源的富mir -218外泌体对骨再生的骨免疫调节作用
目前骨缺损的治疗策略,包括自体移植物、同种异体移植物和传统的生物材料支架,都受到供体部位发病率、免疫排斥和血管化不足的限制。此外,骨缺损中复杂的炎症微环境往往会影响愈合结果,因此需要开发具有增强免疫调节和再生能力的先进生物材料。本研究探讨了黄芪修饰硅酸钙(AstCS)刺激M2巨噬细胞(AstCSM2EVs)的细胞外囊泡在骨再生中的治疗潜力。astcsm2ev通过有效地将巨噬细胞极化至M2表型,表现出优越的免疫调节能力,其特征是显著下调促炎细胞因子(IL-1β, TNF-α),同时上调抗炎介质(IL-4, IL-10)。值得注意的是,AstCSM2EVs表现出增强的血管生成潜能,表现为内皮管形成增加和VEGF分泌增加,同时通过上调ALP、BSP和OC等关键标志物的表达,促进间充质干细胞的成骨分化。机制研究表明,astcsm2ev主要通过mir -218-5p介导的多种信号通路调节这些再生过程,包括nod样受体和ecm受体相互作用通路。在兔股骨缺损模型中,局部给药astcsm2ev显著促进骨再生,表现为增加骨体积分数和改善骨小梁结构,同时有效抑制局部炎症。这些发现表明,astcsm2ev是一种很有前景的骨再生治疗剂,强调了它们在免疫调节、血管生成和成骨方面的多方面作用。本研究介绍了一种结合细胞外囊泡(EV)和免疫调节组织工程策略的创新方法,以改善骨缺损的治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


