Study of photoluminescence sites in Ag-loaded Na–Y type zeolite
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
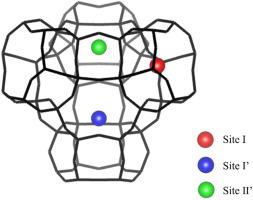
The origin of photoluminescence (PL) was identified as Ag-loaded Na-type Y-type zeolites. To clarify the origin of PL, identifying the luminescent sites of Ag in the zeolite is important. Therefore, we studied the Ag coordination sites when PL development occurs with reduced Ag loading in the zeolite. Using aqueous AgNO3 solutions adjusted to 0.3–1.0 mol/L, we manipulated Ag loading. Quantitative measurements taken using electron probe microanalysis (EPMA) indicated the amounts of Ag atoms per unit cell of the zeolite as 1.3–4.9. The PL measurement results indicated that the PL intensity increased as the Ag loading increased up to 2.0. Furthermore, PL intensity decreased as Ag loading exceeded 2.0. X-ray absorption fine structure (XAFS) measurements showed that up to 2.0 Ag is preferentially coordinated to the center of the hexagonal prisms (Site I) in the zeolite framework. Furthermore, findings indicate that Ag coordinates to the 6-ring (sites I′, II’) in addition to Site I when the number of Ag is greater than 2.0. These results revealed Site I as the luminescence site and sites I′ and II’ as luminescence inhibition sites.
载银Na-Y型沸石的光致发光位点研究
光致发光(PL)的来源确定为负载ag的na型y型沸石。为了弄清PL的来源,确定Ag在沸石中的发光位点是很重要的。因此,我们研究了沸石中银负载减少时PL发育时的银配位位点。利用调节到0.3 ~ 1.0 mol/L的AgNO3水溶液,控制Ag的加载。利用电子探针微量分析(EPMA)进行的定量测量表明,沸石每单位细胞的银原子数量为1.3-4.9。实验结果表明,随着Ag负荷量的增加,发光强度逐渐增大,达到2.0。当Ag加载量超过2.0时,PL强度下降。x射线吸收精细结构(XAFS)测量表明,在沸石骨架中,高达2.0 Ag优先配位到六方棱镜中心(Site I)。结果表明,当Ag数量大于2.0时,除了位点I外,Ag还指向6环(位点I’、II’)。这些结果表明位点I是发光位点,位点I ‘和II ’是发光抑制位点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


