Regulating SLC7A11/GSH/GPX4 axis by glucose dyshomeostasis to simultaneously promote disulfidptosis, cuproptosis and ferroptosis
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
As one of the key targets of tumor metabolic therapy, glucose dyshomeostasis by disrupting glucose metabolism possesses the potential to reverse therapeutic resistance of a variety of regulated cell deaths (RCDs), but the functional pathways are not fully revealed and employed. Herein, we demonstrate that the intervention on SLC7A11/GSH/GPX4 antioxidant axis by glucose dyshomeostasis can simultaneously promote disulfidptosis, cuproptosis and ferroptosis, which is verified by employing glucose oxidase (GOx)-modified copper-apigenin (CuAp) network nanoshuttles (CuAp@GOx NSs) in ovarian tumor therapy. Ap and GOx can jointly induce glucose dyshomeostasis respectively by inhibiting glucose transporter 1-mediated glucose uptake upstream, and consuming massive glucose downstream. As a result of glucose dyshomeostasis, the NADPH supplement is downregulated, which further disrupts SLC7A11/GSH/GPX4 antioxidant axis. This simultaneously boosts disulfidptosis by facilitating cystine accumulation, cuproptosis by attenuating GSH-mediated Cu+ inactivation, and ferroptosis by downregulating GPX4 expression. Owing to the combination of disulfidptosis, cuproptosis and ferroptosis, CuAp@GOx NSs exhibit good efficacy in treating ovarian tumor model. This work proposes an alternative strategy for tumor therapy based on glucose dyshomeostasis, which mainly targets the RCDs relating to SLC7A11/GSH/GPX4 axis.
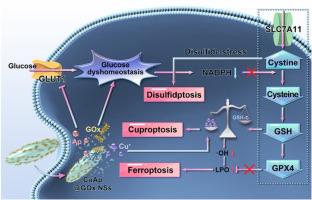
通过葡萄糖失衡调节SLC7A11/GSH/GPX4轴,同时促进二硫下垂、铜下垂和铁下垂
作为肿瘤代谢治疗的关键靶点之一,通过破坏葡萄糖代谢而导致的葡萄糖代谢失调具有逆转多种细胞死亡(rcd)治疗耐药的潜力,但其功能途径尚未被完全揭示和利用。在此,我们证明葡萄糖代谢失调对SLC7A11/GSH/GPX4抗氧化轴的干预可以同时促进二硫下垂、铜下垂和铁下垂,并通过葡萄糖氧化酶(GOx)修饰的铜-芹菜素(CuAp)网络纳米梭(CuAp@GOx NSs)在卵巢肿瘤治疗中得到验证。Ap和GOx分别通过抑制上游葡萄糖转运蛋白1介导的葡萄糖摄取和下游大量消耗葡萄糖,共同诱导葡萄糖代谢失调。由于葡萄糖失衡,NADPH补充下调,进一步破坏SLC7A11/GSH/GPX4抗氧化轴。这同时通过促进胱氨酸积累促进二硫下垂,通过减弱gsh介导的Cu+失活促进铜下垂,通过下调GPX4表达促进铁下垂。由于双重下垂、铜下垂和铁下垂的联合作用,CuAp@GOx NSs在治疗卵巢肿瘤模型中表现出良好的疗效。本研究提出了一种基于葡萄糖失衡的肿瘤治疗替代策略,主要针对SLC7A11/GSH/GPX4轴相关的rcd。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


