Durable 4.5 V sodium metal batteries stabilized by negatively charged metal–organic frameworks in gel polymer electrolyte
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
The development of high-voltage sodium metal batteries faces significant challenges, including high desolvation energy and detrimental interfacial side reactions at elevated voltages. These issues critically impede Na+ transport kinetics and compromise high voltage stability, but can be addressed by strategically modifying the Na+ solvation structure. Herein, we report a metal–organic framework (MOF)-based strategy to reconfigure the Na+ solvation structure specifically for enhanced high-voltage stability and Na+ transport. A composite gel polymer electrolyte (MPVHF) is engineered by incorporating UiO-66-(COONa)2 into a polyvinylidene fluoride-hexafluoropropylene (PVDF-HFP) matrix, followed by liquid electrolyte infiltration. The densely arrayed carboxylate groups (–COO−) on the MOF ligands exert strong electrostatic interactions with Na+, effectively weakening the coordination bonds between Na+ and solvent molecules. This targeted solvation regulation significantly mitigates interfacial side reactions and promotes the formation of a robust, stable electrode–electrolyte interphase crucial for high-voltage operation. Consequently, the MPVHF electrolyte achieves a wide electrochemical stability window extending to 4.93 V and a high Na+ transference number of 0.74. The Na3V2(PO4)2F3 (NVPF)||Na full cells employing MPVHF exhibit stable cycling at 4.5 V cut-off for 1300 cycles at 4 C. This work presents an effective approach to tailor Na+ coordination and transport for high-voltage and fast-charging sodium metal batteries.
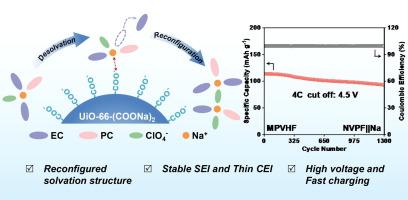
凝胶聚合物电解质中带负电荷的金属有机框架稳定了耐用的4.5 V钠金属电池
高压金属钠电池的发展面临着巨大的挑战,包括高溶解能和高压下有害的界面副反应。这些问题严重阻碍了Na+输运动力学并损害了高压稳定性,但可以通过战略性地修改Na+溶剂化结构来解决。在此,我们报告了一种基于金属有机框架(MOF)的策略来重新配置Na+溶剂化结构,专门用于增强高压稳定性和Na+输运。通过将UiO-66-(COONa)2掺入聚偏氟乙烯-六氟丙烯(PVDF-HFP)基质中,然后进行液体电解质渗透,设计了一种复合凝胶聚合物电解质(MPVHF)。MOF配体上密集排列的羧酸基(-COO−)与Na+产生强烈的静电相互作用,有效地削弱了Na+与溶剂分子之间的配位键。这种有针对性的溶剂化调节显著减轻了界面副反应,促进了一个强大的,稳定的电极-电解质界面相的形成,这对高压操作至关重要。因此,MPVHF电解液具有4.93 V的宽电化学稳定窗口和0.74的高Na+转移数。采用MPVHF的Na3V2(PO4)2F3 (NVPF)||钠离子全电池在4.5 V截止电压下在4℃下稳定循环1300次。该研究为高电压和快速充电钠金属电池定制Na+协调和运输提供了一种有效的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


