Entropy-stabilized oxides with d10 and s0p0 cations as heterostructured photocatalysts with high work function: Experiments and first-principles calculations
IF 14.9
1区 化学
Q1 Energy
引用次数: 0
Abstract
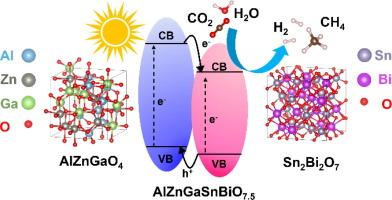
Elements from the right side of the periodic table, including cations with d10 and s0p0 configurations, have been shown to improve photocatalytic activity in various photocatalysts either as dopants or principal elements. This study introduces the first medium- and high-entropy oxide photocatalysts accommodating only d10 and s0p0 cations. The designated oxides, AlZnGaO4 and AlZnGaSnBiO7.5 (dual-phase heterostructure of AlZnGaO4 and 1/2Sn2Bi2O7), demonstrate good optical properties and promising photocatalytic activity for water conversion to hydrogen and CO2 conversion to methane compared to entropy-stabilized cations containing only d0 or d0+d10 configurations. The good activity of these oxides was ascribed to their high work function, which was supported by experimental analysis and first-principles calculations. Moreover, AlZnGaSnBiO7.5 exhibited enhanced activity compared to AlZnGaO4 due to the creation of type II heterojunctions and resultant higher charge carrier separation and lifetime. This study introduces the significance of d10+s0p0 cationic configuration, high work function, and inherent heterojunctions on the design of advanced high-entropy photocatalysts with high photocatalytic activity.
具有d10和s0p0阳离子的熵稳定氧化物作为高功函数异质结构光催化剂:实验和第一性原理计算
元素周期表右侧的元素,包括具有d10和s0p0构型的阳离子,已被证明可以作为掺杂剂或主元素改善各种光催化剂的光催化活性。本研究首次介绍了仅容纳d10和s00阳离子的中、高熵氧化物光催化剂。指定的氧化物AlZnGaO4和AlZnGaSnBiO7.5 (AlZnGaO4和1/2Sn2Bi2O7的双相异质结构)与仅含有d0或d0+d10构型的熵稳定阳离子相比,具有良好的光学性质和良好的光催化活性,可以将水转化为氢和将CO2转化为甲烷。实验分析和第一性原理计算表明,这些氧化物具有较高的功函数,具有良好的活性。此外,与AlZnGaO4相比,AlZnGaSnBiO7.5表现出更强的活性,这是由于II型异质结的产生以及由此产生的更高的电荷载流子分离和寿命。本研究介绍了d10+s0p0阳离子构型、高功函数和固有异质结对设计具有高光催化活性的先进高熵光催化剂的意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


