Glycoursodeoxycholic acid 3 sulfate sodium links hemodynamics and bile acid metabolism in aortic stenosis
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
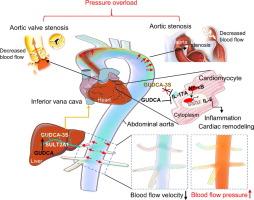
Aortic stenosis (AS) involves aortic obstruction, pressure overload, reduced cardiac output, and impaired organ arterial hemodynamics. Many patients remain at risk of rehospitalization or death after transcatheter aortic valve replacement (TAVR) due to unclear mechanisms. Our previous studies linked bile acids (BAs) metabolism to heart-other organ crosstalk, but the BAs-hemodynamics interplay in AS remains unclear.Objectives
To investigate metabolic abnormalities in AS, focusing on the role of BA metabolism in AS pathogenesis and the underlying mechanisms.Methods
An acute canine model of AS was established via intra-aortic balloon catheter-induced transverse aortic obstruction (ITAO). Computational fluid dynamics (CFD) simulation was performed to assess the arterial hemodynamics of the aorta and other organs. Untargeted/targeted metabolomics and transcriptomics were performed in ITAO and deleting ITAO (deITAO) canines. The findings were validated in 33 controls and 30 AS patients. Metabolic predictive performance was assessed by the area under the receiver-operating characteristic (AUROC) curve. Transcriptomic and western blot analyses were used to assess the effects of glycoursodeoxycholic acid (GUDCA) and glycoursodeoxycholic acid 3 sulfate sodium (GUDCA-3S) on isoproterenol (ISO)-induced myocardial remodeling.Results
ITAO replicated AS hemodynamics (reduced cardiac output, increased aortic velocity), reversed post-deITAO. CFD revealed that ITAO increased organ (e.g., liver) artery pressure, improved after deITAO. Untargeted metabolomics identified 1583 differentially abundant metabolites; transcriptomics revealed 291 DEGs enriched in BA biosynthesis. Targeted BA analysis revealed that GUDCA-3S was elevated in ITAO canines, correlated with aortic velocity (R = -0.4822, P = 0.0002) and BNP (R = 0.3836, P = 0.0019) in AS patients, and exhibited superior AS diagnostic performance (AUROC = 0.844, P < 0.001). Reduced aortic flow upregulated hepatic SULT2A1, driving GUDCA sulfonation to GUDCA-3S and weakening GUDCA’s cardioprotection by impairing IL-17/NF-κB signaling inhibition in ISO-induced cardiomyocytes.Conclusions
BA metabolism dysfunction responds to cardiac hemodynamic changes, with GUDCA-3S linking cardiac hemodynamics and BA metabolism in AS.
甘油去氧胆酸3硫酸钠与主动脉狭窄的血流动力学和胆汁酸代谢有关
主动脉瓣狭窄包括主动脉梗阻、压力超载、心输出量减少和器官动脉血流动力学受损。许多患者在经导管主动脉瓣置换术(TAVR)后仍有再次住院或死亡的风险,原因尚不清楚。我们之前的研究将胆汁酸代谢与心脏-其他器官串扰联系起来,但胆汁酸-血流动力学在AS中的相互作用尚不清楚。目的探讨AS的代谢异常,重点探讨BA代谢在AS发病中的作用及其机制。方法采用主动脉内球囊导管诱导横断主动脉阻塞(ITAO),建立急性AS犬模型。计算流体动力学(CFD)模拟评估主动脉和其他器官的动脉血流动力学。在ITAO和删除ITAO(去ITAO)犬中进行非靶向/靶向代谢组学和转录组学研究。研究结果在33名对照组和30名AS患者中得到了验证。代谢预测性能通过接受者操作特征(AUROC)曲线下面积评估。采用转录组学和western blot方法评价糖胆酸(GUDCA)和糖胆酸3硫酸钠(GUDCA- 3s)对异丙肾上腺素(ISO)诱导的心肌重构的影响。结果实验组的血流动力学(心输出量减少,主动脉流速增加)与对照组完全相同,实验组的血流动力学结果与对照组完全相反。CFD显示,ITAO使脏器(如肝)动脉压升高,去ITAO后有所改善。非靶向代谢组学鉴定出1583种差异丰富的代谢物;转录组学显示291个DEGs富集BA生物合成。有针对性的英航分析显示GUDCA-3S升高ITAO狗,与主动脉速度(R = -0.4822,P = 0.0002)和法国巴黎银行(BNP (R = 0.3836,P = 0.0019)作为病人,和表现出优越的诊断性能(AUROC = 0.844,P & lt; 0.001)。主动脉流量减少可上调肝脏SULT2A1,驱动GUDCA磺化至GUDCA- 3s,并通过损害iso诱导的心肌细胞中IL-17/NF-κB信号抑制而削弱GUDCA的心脏保护作用。结论AS患者BA代谢功能障碍响应心脏血流动力学变化,GUDCA-3S与心脏血流动力学和BA代谢相关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


