Targeting CIRP and IL-6R-mediated microglial inflammation to improve outcomes in intracerebral hemorrhage
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
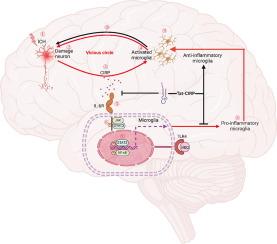
Cold-inducible RNA-binding protein (CIRP) is an emerging inflammatory mediator implicated in neuronal injury following intracerebral hemorrhage (ICH). However, the underlying mechanisms by which CIRP contributes to neuroinflammation remain unclear.Objectives
To investigate the role of neuron-derived CIRP in activating microglial inflammation via IL-6 receptor (IL-6R) signaling after ICH and to evaluate the therapeutic potential of targeting CIRP and IL-6R using a designed peptide inhibitor.Methods
Single-cell RNA sequencing was performed to identify key genes and pathways involved in microglial responses post-ICH. A peptide inhibitor, Tat-CIRP-CMA (TCC), was designed to target CIRP and IL-6R and tested in both in vitro (oxygen-glucose deprivation model) and in vivo (rat ICH model) systems. The effects of TCC on neuronal CIRP release, microglial activation, inflammation, and phagocytic function were assessed. A microglia-specific IL-6Rα knockout mouse model was used to further validate the functional relevance of the IL-6R/ signal transducer and activator of transcription 3 (STAT3) pathway. Clinical analysis of human ICH patients evaluated the correlation between peripheral CIRP expression and infarct volume.Results
Neuron-derived CIRP activates microglia through IL-6R and downstream STAT3 signaling, driving pro-inflammatory responses in ICH. TCC treatment significantly reduced neuronal CIRP release, suppressed microglial activation, and decreased inflammatory cytokine levels. TCC inhibited the IL-6R /STAT3 pathway and enhanced microglial phagocytosis of red blood cells. Microglia-specific IL-6Rα deletion mirrored the anti-inflammatory and neuroprotective effects observed with TCC, reducing hematoma volume and improving sensory and behavioral outcomes. In human ICH patients, elevated peripheral CIRP expression positively correlated with infarct volume, supporting its value as a biomarker for ICH severity and prognosis.Conclusion
CIRP plays a pivotal role in post-ICH neuroinflammation by acting on IL-6R in microglia. The TCC peptide inhibitor effectively reduces CIRP-IL-6R mediated inflammation and supports neuroprotection. CIRP represents a promising therapeutic target and biomarker for ICH.
靶向CIRP和il - 6r介导的小胶质细胞炎症改善脑出血预后
冷诱导rna结合蛋白(CIRP)是一种新兴的炎症介质,与脑出血(ICH)后的神经元损伤有关。然而,CIRP导致神经炎症的潜在机制尚不清楚。目的探讨神经元源性CIRP在脑出血后通过IL-6受体(IL-6R)信号通路激活小胶质细胞炎症中的作用,并评价设计的肽抑制剂靶向CIRP和IL-6R的治疗潜力。方法采用单细胞RNA测序方法,鉴定ich后参与小胶质细胞反应的关键基因和通路。设计了一种肽抑制剂Tat-CIRP-CMA (TCC),以靶向CIRP和IL-6R,并在体外(氧-葡萄糖剥夺模型)和体内(大鼠ICH模型)系统中进行了测试。评估TCC对神经元CIRP释放、小胶质细胞激活、炎症和吞噬功能的影响。利用小胶质细胞特异性IL-6Rα敲除小鼠模型进一步验证IL-6R/信号换能器和转录激活因子3 (STAT3)通路的功能相关性。人类脑出血患者的临床分析评估外周血CIRP表达与梗死体积的相关性。结果神经元源性CIRP通过IL-6R和下游STAT3信号激活小胶质细胞,驱动ICH的促炎反应。TCC治疗显著减少神经元CIRP释放,抑制小胶质细胞激活,降低炎症细胞因子水平。TCC抑制IL-6R /STAT3通路,增强红细胞的小胶质细胞吞噬。小胶质细胞特异性IL-6Rα缺失反映了TCC观察到的抗炎和神经保护作用,减少血肿体积,改善感觉和行为结果。在人类脑出血患者中,外周血CIRP表达升高与梗死体积呈正相关,支持其作为脑出血严重程度和预后的生物标志物的价值。结论cirp通过作用于小胶质细胞IL-6R在脑出血后神经炎症中起关键作用。TCC肽抑制剂有效地减少CIRP-IL-6R介导的炎症并支持神经保护。CIRP是一种有前景的脑出血治疗靶点和生物标志物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


