Integrated network toxicology, machine learning, molecular docking and experimental validation to elucidate mechanism of polyethylene terephthalate microplastics inducing periodontitis
IF 9.7
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Growing evidence highlights the health risks of micro-plastics (MPs) exposure, with reported accumulations in enclosed anatomical sites such as the heart, placenta, and circulatory system. However, the role of MPs in periodontitis remains unexplored. This study employed bioinformatics, network toxicology, machine learning, molecular docking, and experimental approaches to explore the effects and underlying mechanisms of polyethylene terephthalate microplastics (PET-MPs) induced periodontitis. Multi-database screening, including GEO, ChEMBL, STITCH, GeneCards, OMIM, identified 23 candidate targets linked to PET-MPs exposure. Furthermore, we used STRING, Cytoscape software and machine learning approaches to identified 13 core targets. Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment and immune cell infiltration analyses revealed that PET-MPs influence immune-related pathways, such as C-type lectin receptor signaling, VEGF receptor signaling, and TNF signaling. Molecular docking revealed high-affinity binding interactions between PET-MPs and core targets, implicating direct interference with cellular processes. Experimental assays using gingival fibroblasts (GFs) exposed to PET-MPs showed dose-dependent cytotoxicity oxidative stress induction, and pro-inflammatory activation. PET-MPs upregulated inflammation-related markers including Caspase 3, KDR, PIM2, PTGS2, MTOR, MAPK14; while downregulating AKT1 and ALPL. These findings establish a mechanistic framework linking PET-MPs exposure to periodontitis progression via redox-inflammatory crosstalk, offering novel insight into the potential pathogenesis of microplastics on periodontitis.
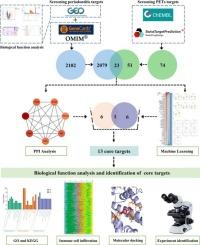
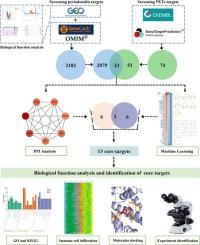
综合网络毒理学、机器学习、分子对接、实验验证等手段,阐明聚对苯二甲酸乙二醇酯微塑料致牙周炎的机制
越来越多的证据强调了微塑料(MPs)暴露的健康风险,据报道,微塑料在心脏、胎盘和循环系统等封闭解剖部位积聚。然而,MPs在牙周炎中的作用仍未被探索。本研究采用生物信息学、网络毒理学、机器学习、分子对接、实验等方法探讨聚对苯二甲酸乙二醇酯微塑料(PET-MPs)诱导牙周炎的作用及其机制。包括GEO、ChEMBL、STITCH、GeneCards、OMIM在内的多数据库筛选确定了23个与PET-MPs暴露相关的候选靶点。此外,我们使用STRING、Cytoscape软件和机器学习方法确定了13个核心靶点。基因本体(GO)、京都基因与基因组百科全书(KEGG)通路富集和免疫细胞浸润分析显示,PET-MPs影响免疫相关通路,如c型凝集素受体信号传导、VEGF受体信号传导和TNF信号传导。分子对接揭示了PET-MPs与核心靶点之间的高亲和力结合相互作用,暗示了对细胞过程的直接干扰。使用暴露于PET-MPs的牙龈成纤维细胞(GFs)进行的实验分析显示出剂量依赖性的细胞毒性、氧化应激诱导和促炎激活。PET-MPs上调炎症相关标志物,包括Caspase 3、KDR、PIM2、PTGS2、MTOR、MAPK14;同时下调AKT1和ALPL。这些发现建立了一个机制框架,将PET-MPs暴露通过氧化还原-炎症串音与牙周炎进展联系起来,为微塑料对牙周炎的潜在发病机制提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


