Targeting EGFR with thiazolidin-4-ones: Structure-guided design, synthesis, and in silico profiling for anticancer drug discovery
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
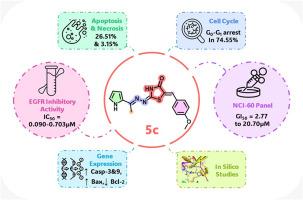
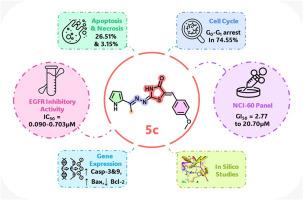
A series of 5-benzylidene-2-hydrazinomethine-thiazolidin-4-one derivatives 5a-y was designed and synthesized based on advanced EGFR TKIs. In vitro EGFR inhibition screening identified twelve compounds with activity comparable to or exceeding that of gefitinib and osimertinib. Thirteen compounds were selected from the NCI for single-dose assay leading to five-dose evaluation of four promising candidates. Compound 5c emerged as the most promising candidate with an IC50 value of 0.090 μM outperforming osimertinib (0.540 μM) and closely matching gefitinib (0.076 μM). In the NCI-60 panel, 5c showed a mean GI% of 84.70 % in single-dose and GI50 values between 2.77 and 20.70 μM in five-dose assays. Cell cycle analysis of 5c revealed G0/G1 arrest in 74.55 % of treated cells versus 58.29 % in controls. Apoptosis reached 26.51 % (vs. 0.65 %) and necrosis of 3.15 % (vs. 1.63 %). Gene expression analysis elicited upregulation of caspase-3 (5.565-fold), caspase-9 (3.549-fold) and Bax (5.029-fold) alongside downregulation of Bcl-2 (0.356-fold). Against mutant EGFR forms, 5c maintained activity with IC50s between 0.147 and 0.703 μM. Molecular docking supported this activity showing favorable binding energies compared to gefitinib. Compound 5c met Lipinski's, Veber's and Eagan's criteria for oral bioavailability. ADME and toxicity profiles suggested a safer profile than both gefitinib and osimertinib, including lower immunotoxicity (0.74 vs. 0.99) and negligible hepatotoxic, neurotoxic and respiratory risks. These findings highlight 5c as a selective and potent EGFR TKI with strong therapeutic potential.
用噻唑烷-4- 1靶向EGFR:结构导向设计、合成和抗癌药物发现的硅谱分析
以先进的EGFR TKIs为基础,设计合成了一系列5-苄基-2-肼甲胺-噻唑烷-4- 1衍生物5a-y。体外EGFR抑制筛选鉴定出12种活性与吉非替尼和奥西替尼相当或超过的化合物。从NCI中选择了13种化合物进行单剂量试验,然后对4种有希望的候选药物进行五剂量评估。化合物5c的IC50值为0.090μM,优于奥西替尼(0.540μM),与吉非替尼(0.076μM)非常接近。在NCI-60面板中,5c显示单剂量时的平均GI%为84.70%,五剂量时的GI50值在2.77-20.70μM之间。细胞周期分析显示,74.55%的处理细胞G0/G1阻滞,而对照组为58.29%。细胞凋亡26.51% (vs. 0.65%),坏死3.15% (vs. 1.63%)。基因表达分析显示caspase-3(5.565倍)、caspase-9(3.549倍)和Bax(5.029倍)上调,Bcl-2(0.356倍)下调。对于突变型EGFR, 5c与ic50在0.147-0.703μM之间保持活性。与吉非替尼相比,分子对接支持这种活性,显示出更有利的结合能。化合物5c符合Lipinski’s、Veber’s和Eagan’s口服生物利用度标准。ADME和毒性分析表明,ADME比吉非替尼和奥西替尼更安全,包括更低的免疫毒性(0.74比0.99)和可忽略的肝毒性、神经毒性和呼吸风险。这些发现强调了5c作为一种选择性和有效的EGFR TKI具有很强的治疗潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


