Microglia-astrocyte crosstalk regulates synapse remodeling via Wnt signaling
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
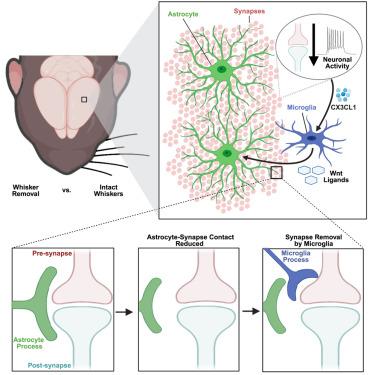
Astrocytes and microglia are emerging key regulators of activity-dependent synapse remodeling that engulf and remove synapses in response to changes in neural activity. Yet, the degree to which these cells communicate to coordinate this process remains an open question. Here, we use whisker removal in postnatal mice to induce activity-dependent synapse removal in the barrel cortex. We show that astrocytes do not engulf synapses in this paradigm. Instead, astrocytes reduce contact with synapses prior to microglia-mediated synapse engulfment. We further show that the reduced astrocyte-synapse contact is dependent on the release of Wnts from microglia downstream of neuron-to-microglia fractalkine ligand-receptor (CX3CL1-CX3CR1) signaling. These results demonstrate an activity-dependent mechanism by which microglia instruct astrocyte-synapse interactions, providing a permissive environment for microglia to remove synapses. We further show that this mechanism is critical to remodel synapses in a changing sensory environment and that this signaling is upregulated in several disease contexts.
小胶质细胞-星形胶质细胞串扰通过Wnt信号调节突触重塑
星形胶质细胞和小胶质细胞是活动依赖性突触重塑的关键调节因子,在神经活动变化的反应中吞噬和移除突触。然而,这些细胞沟通协调这一过程的程度仍然是一个悬而未决的问题。在这里,我们在出生后的小鼠中使用须去除来诱导桶状皮层中活动依赖的突触去除。我们发现星形胶质细胞在这种情况下不会吞噬突触。相反,星形胶质细胞在小胶质细胞介导的突触吞噬之前减少了与突触的接触。我们进一步表明,星形胶质细胞-突触接触的减少依赖于神经元到小胶质细胞fractalkine配体受体(CX3CL1-CX3CR1)信号传导下游小胶质细胞释放的wnt。这些结果证明了一种活动依赖机制,通过这种机制,小胶质细胞指导星形胶质细胞与突触的相互作用,为小胶质细胞移除突触提供了一个允许的环境。我们进一步表明,这种机制对于在不断变化的感觉环境中重塑突触至关重要,并且这种信号在几种疾病背景下上调。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


